Current HIV treatments need to be taken for life by those infected as antiretroviral therapy is unable to eliminate viral reservoirs lurking in immune cells. Institut Pasteur scientists have identified the characteristics of CD4 T lymphocytes that are preferentially infected by the virus – it is their metabolic (or energy-producing) activity1 that enables the virus to multiply. Thanks to metabolic activity inhibitors, the researchers have managed to destroy these infected cells, or "reservoirs", ex vivo. Their findings were published in the journal Cell Metabolism on December 20, 2018.
Current HIV treatments need to be taken for life by those infected as antiretroviral therapy is unable to eliminate viral reservoirs lurking in immune cells. Institut Pasteur scientists have identified the characteristics of CD4 T lymphocytes that are preferentially infected by the virus – it is their metabolic (or energy-producing) activity1 that enables the virus to multiply. Thanks to metabolic activity inhibitors, the researchers have managed to destroy these infected cells, or "reservoirs", ex vivo. Their findings were published in the journal Cell Metabolism on December 20, 2018.
The antiretroviral treatment used today is designed to block HIV infection but it is not able to eliminate the virus from the body. The virus remains in reservoirs – the CD4 T lymphocyte immune cells, the main targets of HIV. However, the virus does not infect all types of CD4 cell and until now the reason for this was not well known. In this study, scientists from the HIV, Inflammation and Persistence Unit at the Institut Pasteur and colleagues have identified the characteristics of the different CD4 subpopulations, which are associated with HIV infection.
The more the CD4 cells are differentiated, or experienced, the more they need to produce energy to perform their function. Experiments have shown that it is the metabolic activity of the cell, and in particular its glucose consumption, that plays a key role in susceptibility to HIV infection. The virus primarily targets cells with high metabolic activity. To multiply, it hijacks the energy and products provided by the cell.
This requirement constitutes a weakness for the virus and could be exploited to tackle infected cells. Scientists succeeded in blocking the infection ex vivo thanks to metabolic activity inhibitors that have already been investigated in cancer research.
We have observed ex vivo that, thanks to certain metabolic inhibitors, the virus is no longer able to infect cells and amplification is halted in reservoirs of patients receiving antiretroviral treatment.

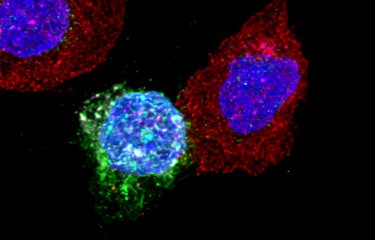
Schematic representation of CD4 T lymphocytes (in purple). Left: the CD4 T lymphocyte with significant metabolic activity is infected with HIV (in green). Right: the CD4 T lymphocyte has no metabolic activity and therefore cannot be infected. © Institut Pasteur / Nicolas Huot
This research opens new ways towards possible remission through the elimination of reservoir cells. The next research phase will involve assessing the potential of these metabolic inhibitors in vivo.
This study was funded by the Institut Pasteur, AmfAR (American Foundation for AIDS research) and Sidaction.
[1] Metabolic (energy) activity of a cell. It is the cell's molecule synthesis and breakdown activities that enable it to produce energy. There are numerous metabolic pathways. Glycolysis, for example, is when sugar is turned into energy.
Cellular metabolism is a major determinant of HIV-1 reservoir seeding in CD4+ T cells and offers an opportunity to tackle infection, Cell Metabolism, December 20, 2018
José Carlos Valle-Casuso (1), Mathieu Angin1, Stevenn Volant (2), Caroline Passaes (1), Valérie Monceaux (1), Anastassia Mikhailova (1), Katia Bourdic (3), Véronique Avettand-Fenoel (4,5), Faroudy Boufassa (6), Marc Sitbon (7), Olivier Lambotte (3,8), Maria-Isabel Thoulouze (9), Michaela Müller-Trutwin (1), Nicolas Chomont (10) and Asier Sáez-Cirión (1)
(1) Institut Pasteur, Unité HIV Inflammation et Persistance, Paris, France
(2) Institut Pasteur, Hub Bioinformatique et Biostatistique – C3BI, USR 3756 IP CNRS – Paris, France
(3) Assistance Publique Hôpitaux de Paris, Hôpital Bicêtre, Service de Médecine Interne et Immunologie clinique, 94275 Le Kremlin-Bicêtre, France
(4) Université Paris Descartes, Sorbonne Paris Cité, EA7327, Paris, France.
(5) Assistance Publique Hôpitaux de Paris, CHU Necker-Enfants, Laboratoire de Virologie, Malades, Paris, France
(6) INSERM U1018, Centre de recherche en Epidémiologie et Santé des Populations, Université Paris Sud, Le Kremlin Bicêtre, France
(7) Institut de Génétique Moléculaire de Montpellier, University of Montpellier, CNRS, Montpellier,
France
(8) CEA, Université Paris Sud, INSERM U1184, Immunology of Viral Infections and Autoimmune Diseases (IMVA), IDMIT Department / IBFJ, Fontenay-aux-Roses, France
(9) Institut Pasteur, Unité Virologie Structurale, Paris, France
(10) Centre de Recherche du CHUM and Department of microbiology, infectiology and immunology, Université de Montréal, H2X0A9, Canada