Developing effective therapeutic vaccines requires knowing how to chemically recognize microbes, as well as exposing them to treatment. Able to mutate very quickly and to camouflage itself inside the cells it infects, the AIDS virus (or HIV) constitutes a challenge for researchers.
| This article is the second in a series devoted to the hopes of HIV-AIDS research, on the occasion of the 40th anniversary of the identification of the virus. |
 The HIV virus is microscopic, measuring more than a hundred times smaller than a cell. Like all viruses, it requires a host cell to replicate, but HIV does not infect just any cells in the body. Instead, it targets cells in the immune system, such as CD4 T cells and macrophages. The virus binds to CD4 surface proteins and releases a capsid containing two identical RNA strands into the cytoplasm of the cell. Similar to all retroviruses, the RNA of HIV must undergo reverse transcription into DNA to be integrated into the host cell’s genome, allowing it to replicate and coexist with the host. From that point on, each time the cell divides, the virus is inherited by the daughter cells, giving it a highly effective Trojan horse. In summary, this is how HIV replicates.
The HIV virus is microscopic, measuring more than a hundred times smaller than a cell. Like all viruses, it requires a host cell to replicate, but HIV does not infect just any cells in the body. Instead, it targets cells in the immune system, such as CD4 T cells and macrophages. The virus binds to CD4 surface proteins and releases a capsid containing two identical RNA strands into the cytoplasm of the cell. Similar to all retroviruses, the RNA of HIV must undergo reverse transcription into DNA to be integrated into the host cell’s genome, allowing it to replicate and coexist with the host. From that point on, each time the cell divides, the virus is inherited by the daughter cells, giving it a highly effective Trojan horse. In summary, this is how HIV replicates.

Immune cells provide the virus with a sort of Trojan horse that enables it to reach environments in which it either replicates actively or persists silently, without needing to do anything.
Asier Sáez-Cirión Head of the Viral reservoirs and immune control unit
But our knowledge of HIV is constantly evolving, and we are realizing that the mechanisms that regulate and influence HIV entry and replication in cells are still poorly understood. If we are to develop effective drugs or even try to produce a vaccine, it is crucial that we understand the pathogenesis of the virus, as Françoise Barré-Sinoussi, one of the scientists who discovered HIV, soon concluded. This is why the Institut Pasteur’s teams are pursuing their research, and they are constantly making new discoveries. For example, the team headed by Elisabeth Menu, leader of the Mucosal Immunity and Sexually Transmitted Infection Control (MISTIC) group, is investigating the factors that regulate local inflammation (the menstrual cycle, microbiota composition, co-infections, exposure to seminal fluid, etc.) and influence susceptibility to HIV-1 in the mucosa of the female reproductive system. The team led by Francesca Di Nunzio, Head of the Advanced Molecular Virology Unit, recently demonstrated that the HIV capsid can pass through the pores of the host cell nucleus, and that the viral RNA genome accumulates in the nucleus.
Inside infected cells
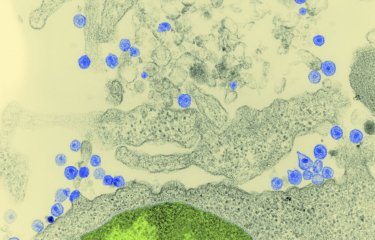
«We are investigating the early stages of infection as it is becoming clear that they are crucial for the establishment of viral reservoirs, and the persistence of these reservoirs is a major obstacle to HIV cure», explains Francesca Di Nunzio. «And nowadays we are able to observe living cells containing the HIV genome, taken directly from infected humanized mice from James Di Santo’s laboratory (the Innate Immunity Unit).» Using a patented fluorescence microscopy method, Francesca Di Nunzio’s team is able to monitor the evolution of viruses in the different cell compartments in vivo, in different organs. The scientists have demonstrated that uncoating and reverse transcription occur in the nucleus, and not in the cytoplasm as previously thought. «We also observed that when a cell is invaded by several viruses, the viral RNA migrates to the nucleus and aggregates into highly dynamic «membraneless organelles» (HIV MLOs)», explains Francesca Di Nunzio.
Microscopy images show small puncta containing viral genomic RNA in the host nucleus, which group together over time to form large clusters where budding of viral DNA occurs. «This is the first demonstration that RNA travels to the nucleus, that it is concentrated inside membraneless organelles for reverse transcription into DNA before being integrated into the cell genome», marvels Francesca Di Nunzio. And the images are astonishing as they actually show spheres measuring a few hundred nanometers encapsulating viruses from the beginning of an infection in the nucleus of living cells. Since these organelles persist in some cells, they could potentially serve as markers for identifying infected cells in the viral reservoir. Moreover, they may represent a promising target for future treatments. For HIV is indeed a master of Camouflage...

I have always found this virus fascinating. It is one of the «smartest» in the way it uses human cells.
Francesca Di Nunzio Head of the Advanced molecular virology laboratory
Targeting HIV reservoirs
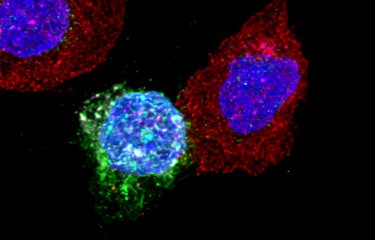
When viral DNA is «active», it literally takes control of the infected cell and prompts it to produce thousands of copies of the virus. Viral particles produced in huge numbers – especially in the early stages of infection – bud from the surface of the hijacked cell, then invade neighboring cells. Research by the team led by Olivier Schwartz, Head of the Virus and Immunity Unit, has demonstrated that the virus essentially spreads via connections between immune cells. «The virus uses these synapses to spread effectively from one cell to another without being detected by antibodies», explains Olivier Schwartz.
But sometimes the viral DNA remains dormant, and the latent virus stays «camouflaged» inside some immune cells, without causing any damage but unable to be eliminated either by the immune system or by antiretroviral therapy. This is a major challenge of research at the Institut Pasteur and in laboratories worldwide: how to locate HIV reservoirs, or in other words how to identify the cells in which the dormant virus is hiding so that they can be destroyed and lead to remission or even a cure for HIV infection. «We still need to understand the mechanisms by which the reservoir is formed, maintained and reactivated when treatment is stopped», says Olivier Schwartz. «Broadly neutralizing antibodies represent a promising avenue for reducing or eliminating infected cells. We have shown that antibodies act not only by neutralizing viral particles and preventing new cells from being infected, but also by blocking the release of viruses produced by infected cells and facilitating the destruction of these cells.»
The immune system in actionWhen dealing with an infectious agent, the body has two types of defense mechanism: innate immunity, which is immediate, and adaptive immunity, which kicks in later but lasts longer. Innate immunity involves several cell types (macrophages, dendritic cells, NK cells, etc.) and protein types (cytokines, interferons, etc.). Following the interaction between infectious agents and innate immunity, adaptive immunity kicks into action in the lymphoid organs. The infectious agent is captured by specialized cells which stimulate B and CD8 T cells, with the help of CD4 T cells. B cells then produce specific antibodies against the infectious agent, while CD8 cells become capable of recognizing and destroying infected cells. |
Extreme diversity
Another characteristic of interest to researchers is the remarkable genetic diversity of HIV, for which there are two main types: HIV-1 and HIV-2. In a single infected individual receiving no treatment, HIV can generate more diversity than during a worldwide influenza epidemic. This is because HIV makes multiple mistakes while copying RNA into DNA, giving rise to thousands of variants with slight differences. So the virus varies not just from one individual to the next, but also from one cell to the next.
This means that a person with HIV needs to defend themselves from a huge number of different viruses, even if they were initially only infected by a single virus. And if the infection is not rapidly brought under control with treatment, the task soon becomes impossible. The virus evolves so quickly that the anti-HIV cells or antibodies produced are ultimately of no use. The immune system ends up running out of steam. This extreme variability, and the need to bring the virus under control quickly before it develops resistance to drugs, led to the development of triple therapies. The diversity of HIV is also what makes it so complicated to develop a vaccine – even the influenza virus, which is also skilled in the art of metamorphosis, requires a new vaccine each year.