Phase 1 trial of the vaccine candidate which was developed under an R&D collaboration between Themis Bioscience GmbH and the Institut Pasteur (Paris) shows good immunogenicity, safety and tolerability.
Themis Bioscience (‘Themis’), a biotechnology company developing innovative prophylactic vaccines for emerging tropical infections, and the Institut Pasteur, an international biomedical research center based in Paris (France) today announced the publication of the phase I study results for a recombinant measles-virus-based chikungunya vaccine (MV-CHIK) in The Lancet Infectious Diseases. The study was performed in collaboration with the Department of Clinical Pharmacology at the Medical University of Vienna and the Viral Diseases Branch of the Walter Reed Army Institute of Research (WRAIR) in the USA. The peer-reviewed article is entitled “Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial”.
Press release
Vienna, Austria, March 2, 2015
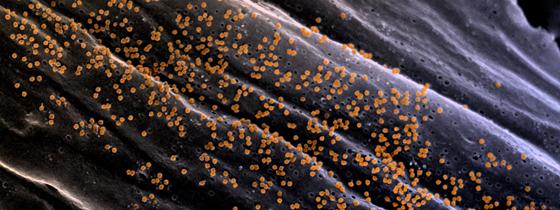
Chikungunya fever is a mosquito-borne viral disease causing symptoms including fever, headache, joint and muscle pain and bleeding of the nose and gums. Importantly, a large number of infected patients suffer from chronic sequela months and years after the acute infection. The chikungunya virus originated in Asia and western and central Africa and rising levels of travel and global warming led to increasing incidences of the disease in temperate zones, thus becoming a global health threat. Since late 2013, more than one million cases have been reported in the Americas and the Caribbean alone, resulting in a significant public health and economic burden.
Themis’ recombinant measles-chikungunya vaccine phase I study was conducted between November 2013 and June 2014 with a total of 42 healthy male and female individuals from age 18-45 being randomised into 4 cohorts for this dose escalation study. Subjects were administered one injection with either a low, medium or high dose of the chikungunya vaccine or the active comparator Priorix (standard measles vaccine). The study investigated the immunogenicity, safety and tolerability of the vaccine. In addition, randomized patients received a booster injection on either day 28 or day 90 after the first vaccination.
The candidate vaccine raised concentrations of neutralising antibodies to chikungunya in all dose cohorts after one immunisation, with seroconversion* rates of patients producing anti-chikungunya antibodies of 44% in the low, 92% in the medium, and 90% in the high-dose group. The immunogenicity of the candidate vaccine was not affected by pre-existing anti-measles immunity. The second vaccination resulted in a 100% seroconversion for all patients in the candidate vaccine groups. The candidate vaccine had an overall good safety profile, and while the rate of adverse events increased with vaccine dose and volume, no vaccination-related serious adverse events were recorded.
Dr. Frederic Tangy, head of the Viral Genomics and Vaccination Unit at the Institut Pasteur (Institut Pasteur, CNRS UMR-3569), who developed this vaccine technology, explained: “The measles vaccine has already proven its high efficacy and safety on more than a billion vaccinated individuals during the last 30-40 years. Therefore, this platform offers an excellent safety profile and the clear advantage of a validated and easy manufacturing process. The present result demonstrates that a measles vector can be used in the presence of pre-existing immunity to measles, likely because it is a replicating vector. This gives another great advantage to this vaccine strategy.”
“Recent outbreaks have raised awareness of chikungunya virus worldwide and whilst further work is needed to show safety, tolerability, and ability of the vaccine to protect against live chikungunya virus, our trial data suggest that this novel vaccine is an excellent candidate to help address this urgent medical need”, explains Dr. Erich Tauber, CEO of Themis. “With these promising results we are advancing the chikungunya vaccine programme and aim to move rapidly into phase II studies.”
* seroconversion rate: percentage of vaccinated persons that produce antibodies
About Themis
Themis Bioscience GmbH develops prophylactic vaccines with a focus on emerging tropical infectious diseases and has initial vaccine candidates currently in development for chikungunya and dengue fever. The company has exclusive, worldwide licenses for chikungunya- and dengue vaccines, based on the innovative and fully patent-protected measles virus vaccine vector platform from the Institut Pasteur in Paris. This platform underpins Themis’ growing pipeline of vaccines. Themis and Institut Pasteur are actively collaborating on additional targets. Themis was founded by experienced vaccine experts in September 2009 and is based in Vienna. For more information, visit the website: www.themisbio.com
About Institut Pasteur
Louis Pasteur created the Institut Pasteur in 1887 as a private non-profit foundation that rapidly became world-renowned for its biomedical research. The main aim of the Institut Pasteur is understanding and preventing diseases throughout the world through excellent scientific and public health research, teaching and other activities. Together with its major contribution to a deeper understanding of fundamental aspects of life, the Institut Pasteur continues to devote a large part of its efforts to infectious diseases, inherited disorders, neurodegenerative diseases and certain cancers. Close to 2,400 people work on its main campus in Paris, which is at the heart of an international network of 32 research institutes on 5 continents. Over the years, 10 Institut Pasteur researchers have received the Nobel Prize. www.pasteur.fr
About the vaccine technology
The core technology of the measles vector platform has been developed at the Institut Pasteur in Paris and is licensed to Themis. It relies on the use of the standard measles vaccine as a vaccination vector. Genes coding for selected antigens from the chikungunya virus have been inserted into the genome of this well-established vaccine. The measles-chikungunya vaccine delivers the chikungunya antigens directly to macrophages and dendritic cells - the most potent and effective antigen-presenting cells, thereby triggering a specific immune response to chikungunya virus. This results in a powerful, antigen-focused immune response, which is most likely to confer long-term immunity as do measles vaccine.
Illustration: Viral particles on the cell surface of Chikungunya virus-infected fibroblasts. © Institut Pasteur/Thérèse Couderc et Marc Lecuit, unité de Biologie des Infections Inserm U1117
Source
Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial, The Lancet Infectious Diseases, published online March 2th, 2015.
Katrin Ramsauer, PhDb,†, Michael Schwameis, MDa,†, Christa Firbas, MDa, Matthias Müllner, PhDb, Robert J Putnak, PhDe, Stephen J Thomas, MDe, Philippe Desprèsd, Erich Tauber, MDb, Dr Bernd Jilma, MDa, Frederic Tangy, PhDc
a Department of Clinical Pharmacology, Medical University of Vienna, Austria, Europe
b Themis Bioscience GmbH, Vienna, Austria
c Viral Genomics and Vaccination Unit, Institut Pasteur, Paris, CNRS UMR-3569, France
d Department of Infections and Epidemiology, Institut Pasteur, La Réunion, UMR U1187 PIMIT (I2T), France
e Walter Reed Army Institute of Research, Viral Diseases Branch, Silver Springs, MD, USA
† These authors contributed equally.