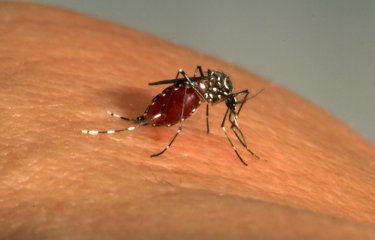
What factors facilitate the emergence of a previously unknown virus in the human population? Scientists from the CNRS, the Institut Pasteur and the IRD, in collaboration with the Institut Pasteur International Network and with several other institutions worldwide, have helped answer that question for the Zika virus. Their study sheds light on regional and continental disparities in Zika outbreaks and explains why there have been no major outbreaks in Africa.
The Zika virus has spread across the planet over the past decade, causing millions of infections, some of which are associated with congenital malformations and neurological disorders (see the Institut Pasteur's Zika fact sheet).
Scientists from the CNRS, the Institut Pasteur (Paris), the Institut Pasteur International Network, and the IRD* set out to investigate the primary vector of the Zika virus, the Aedes aegypti mosquito. Between 5,000 and 10,000 years ago, this mosquito species native to Africa gave rise to a human-adapted subspecies that spread to other continents during the last centuries.
Aedes aegypti, a discreet but formidable mosquito
Did you know that the Earth is home to no fewer than 3,546 species of mosquito? Only about a hundred of these feed on humans. Of these, two species – Aedes aegypti and its alter ego Aedes albopictus (or tiger mosquito) – are notable for their capacity to carry arboviruses, which are responsible for diseases such as yellow fever, dengue, chikungunya and Zika virus disease that kill about 60,000 people every year. Mosquitoes of the genus Aedes are less obtrusive than their cousins, the Anopheles mosquitoes, which carry malaria (the cause of 435,000 deaths in 2015), but they are no less of a problem and are now present almost everywhere in the world – including in France, with Aedes albopictus found in mainland France and Aedes aegypti in the overseas territories and departments on the American continent.
The invasive subspecies of Aedes aegypti, widespread outside Africa, is very effective at transmitting the Zika virus
The invasive subspecies of Aedes aegypti is believed to have become a major vector of viruses, including those responsible for yellow fever and dengue, because of its marked preference for human blood.
By experimentally comparing wild populations of Ae. aegypti from various regions of the globe, the scientists realized that the invasive subspecies is very effective at transmitting the Zika virus, not only because it has more frequent contacts with humans for blood meals, but also as a result of its greater susceptibility to the virus relative to the African subspecies.
An explanation for the absence of a major Zika outbreak in Africa
One of the keys to the scientists' discovery was examining "hybrid" populations between the two subspecies that exist naturally in West Africa and have demonstrated intermediate susceptibility to the Zika virus. The invasive subspecies of the Ae. aegypti mosquito is able to transmit the Zika virus so effectively not only because of its frequent contacts with humans when taking blood meals, but also because it is more susceptible to infection with the Zika virus, compared to the African subspecies.
This also helps explain why there has never been a major Zika virus outbreak in Africa, where the native subspecies of Ae. aegypti is less capable of transporting the virus. These results shed new light on the emergence of the Zika virus and the regional and continental disparities in its impact on public health.
* The researchers hail from the Evolutionary Genomics, Modeling, and Health unit (CNRS / Institut Pasteur), MIGEVEC (CNRS / IRD / University of Montpellier), the Mouse Genetics Laboratory (Institut Pasteur), the Pasteur Institutes of French Guiana, Guadeloupe, Cambodia and Dakar, the QIMR Berghofer Medical Research Institute (Australia), the Princeton University (USA), the Fundación Universidad del Norte (Colombia), the Centre Interdisciplinaire de Recherches Médicales de Franceville (Gabon), the Uganda Virus Research Institute (Uganda), the MRC-University of Glasgow Centre for Virus Research (UK), the Armed Forces Research Institute of Medical Sciences (Thailand), the Université Cheikh Anta Diop (Senegal), the Kenya Medical Research Institute (Kenya), the Centers for Disease Control and Prevention (USA), the Institut Louis Malardé (French Polynesia), and the Walter Reed Army Institute of Research (USA).
Source
Enhanced Zika virus susceptibility of globally invasive Aedes aegypti populations, Science, November 20, 2020
Fabien Aubry1, Stéphanie Dabo1, Caroline Manet2, Igor Filipović3, Noah H. Rose4,5, Elliott F. Miot1,6, Daria Martynow1, Artem Baidaliuk1,6, Sarah H. Merkling1, Laura B. Dickson1, Anna B. Crist1, Victor O. Anyango1, Claudia M. Romero-Vivas7, A 5 nubis Vega-Rúa8, Isabelle Dusfour9, Davy Jiolle10,11, Christophe Paupy10,11, Martin N. Mayanja12, Julius J. Lutwama12, Alain Kohl13, Veasna Duong14, Alongkot Ponlawat15, Massamba Sylla16, Jewelna Akorli17, Sampson Otoo17, Joel Lutomiah18, Rosemary Sang18, John-Paul Mutebi19, Van-Mai Cao-Lormeau20, Richard G. Jarman21, Cheikh T. Diagne22, Oumar Faye22, Ousmane Faye22, Amadou10 A. Sall22, Carolyn S. McBride4,5, Xavier Montagutelli2, Gordana Rašić3, Louis Lambrechts1*
1. Insect-Virus Interactions Unit, Institut Pasteur, CNRS UMR2000, Paris, France
2. Mouse Genetics Laboratory, Institut Pasteur, Paris, France 15
3. Mosquito Control Laboratory, QIMR Berghofer Medical Research Institute, Brisbane, Australia
4. Department of Ecology & Evolutionary Biology, Princeton University, Princeton, New Jersey, United States of America
5. Princeton Neuroscience Institute, Princeton University, Princeton, New Jersey, United States of America
6. Collège doctoral, Sorbonne Université, Paris, France
7. Laboratorio de Enfermedades Tropicales, Departamento de Medicina, Fundación Universidad del Norte, Barranquilla, Colombia
8. Institut Pasteur de la Guadeloupe, Laboratory of Vector Control Research, Transmission Reservoir and Pathogens Diversity Unit, Morne Jolivière, Guadeloupe, France
9. Vector Control and Adaptation, Institut Pasteur de la Guyane, Vectopole Amazonien Emile Abonnenc, Cayenne, French Guiana
10. MIVEGEC, Montpellier University, IRD, CNRS, Montpellier, France
11. Centre Interdisciplinaire de Recherches Médicales de Franceville, Franceville, Gabon
12. Department of Arbovirology, Uganda Virus Research Institute, Entebbe, Uganda
13. MRC-University of Glasgow Centre for Virus Research, Glasgow, United Kingdom
14. Virology Unit, Institut Pasteur du Cambodge, Phnom Penh, Cambodia
15. Department of Entomology, Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand
16. Unité d’Entomologie, de Bactériologie, de Virologie, Département de Biologie Animale, Faculté des Sciences et Techniques, Université Cheikh Anta Diop, Dakar, Sénégal
17. Department of Parasitology, Noguchi Memorial Institute for Medical Research, University of Ghana, Accra, Ghana
18. Arbovirus/Viral Hemorrhagic Fevers Laboratory, Center for Virus Research, Kenya Medical Research Institute, Nairobi, Kenya
19. Centers for Disease Control and Prevention, Fort Collins, Colorado, United States of America
20. Institut Louis Malardé, Papeete, Tahiti, French Polynesia
21. Viral Diseases Branch, Walter Reed Army Institute of Research, Silver Spring, Maryland, United States of America
22. Institut Pasteur Dakar, Arbovirus and Viral Hemorrhagic Fevers Unit, Senegal
This study is part of the priority scientific area Emerging infectious diseases of the Institut Pasteur's strategic plan for 2019-2023.
For more information, please visit