A series of human genome-wide studies have revealed a strong correlation between a chromosomal locus and the risk of developing tobacco dependence. One mutation has been identified in particular (rs16969968, or SNPα5). Studies have investigated whether this mutation is also associated with alcoholism, given the known high comorbidity between tobacco and alcohol addiction. A team from the Institut Pasteur and the CNRS (French National Center for Scientific Research) recently observed that rats carrying the SNPα5 mutation have an increased appetite for alcohol. This article explores their research.
"Nicotine, which is responsible for most of the addictive properties of tobacco, alters brain function by binding to nicotinic acetylcholine receptors", points out Morgane Besson, a researcher in the Institut Pasteur Integrative Neurobiology of Cholinergic Systems Unit, led by Uwe Maskos. These receptors are transmembrane channel proteins made up of five subunits designated α and β, which can associate in multiple different combinations. A series of human genome-wide studies have revealed a strong correlation between chromosomal locus 15 (15q25), containing nicotinic subunit genes α5, α3 and β4, and the risk of developing tobacco dependence. One mutation in particular has been identified in nicotinic subunit gene α5 (SNPα5), leading to an alteration in the protein sequence and a two-fold risk of tobacco dependence in homozygous carriers.
A supposed link between tobacco and alcohol dependence
Since there is a very high level of comorbidity between tobacco dependence and alcoholism and they represent the two main causes of premature death, studies have since investigated whether this mutation is also associated with alcoholism. "Some data collected suggested that this is indeed the case but it is difficult to draw conclusions from these human studies given the conflicting findings", explains Morgane Besson. "We therefore attempted to characterize the link between this mutation and alcohol dependence further, by subjecting rats that carry this mutation – produced in the lab – to a procedure known as ethanol self-administration." During this procedure, the animals are free to voluntarily consume an ethanol solution (or not) by pressing a lever to receive a drop.
An increased appetite for alcohol observed
"We observed that rats carrying the mutation have a more marked appetite for alcohol than the control rats, and exhibit increased relapse to alcohol seeking after abstinence, in association with hyperactivation of the insular cortex, a region crucial for interoception." The term interoception refers to an individual's ability to accurately appraise internal bodily signals, i.e. physiological activity such as heart rate, hunger or pain, determining a state of comfort or discomfort that influences emotions and behavior.
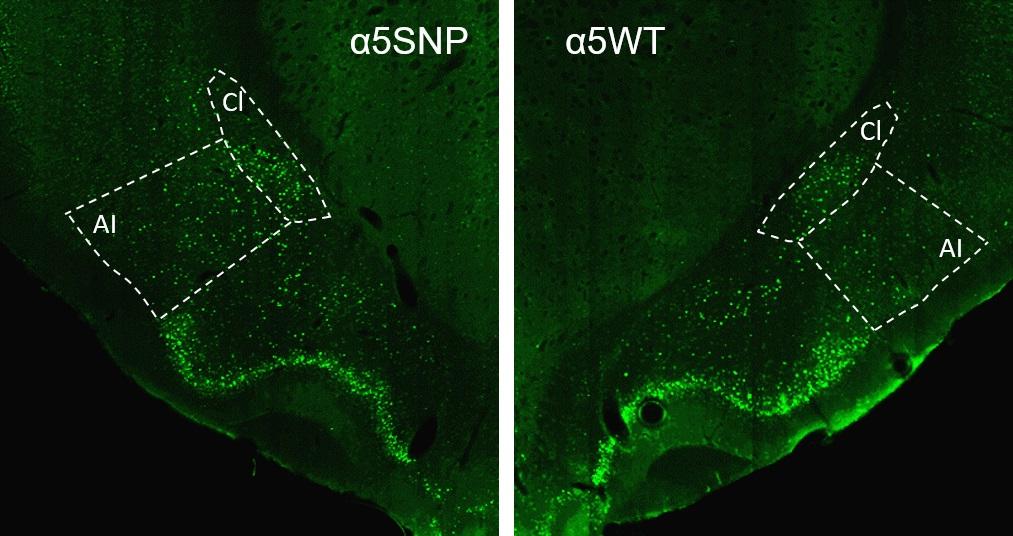
In addition, the SNPα5 mutation has been associated with a higher body mass index in non-smokers but, conversely, a lower BMI in smokers. "We have shown that rats carrying this mutation also have a greater appetite for food, and a higher rate of relapse regarding the consumption of particularly appetizing food, mirroring their behavior with nicotine and alcohol", continues Morgane Besson. Finally, the scientists observed that nicotine replacement therapy reduces the likelihood of consuming this food again. The data suggests that, in addition to a direct impact on the effects of nicotine on the brain, this mutation influences the risk of dependence on several drugs and possibly of eating disorders, but this still needs to be assessed in humans.
Molecules that specifically target the activity of nicotinic receptors containing the α5 subunit could be a new therapeutic target of interest in individuals carrying this mutation.
Source
Profound alteration in reward processing due to a human polymorphism in CHRNA5: a role in alcohol dependence and feeding behavior, Neuropsychopharmacology, July 9, 2019
Morgane Besson1, Benoît Forget1,2, Caroline Correia1,3, Rodolphe Blanco1 and Uwe Maskos1
1. Département de Neuroscience, unité de Neurobiologie Intégrative des Systèmes Cholinergiques, CNRS UMR 3571, Institut Pasteur, 25 rue du Dr Roux, 75015 Paris, France
2. Present address: Sorbonne Universités, UPMC Université Paris 06, INSERM, CNRS, Neuroscience Paris Seine - Institut de Biologie Paris Seine (NPS - IBPS), 75005 Paris, France
3. Present address: Laboratoire de Neurosciences Cognitives et Adaptatives, CNRS UMR 7364, Université de Strasbourg, 67000 Strasbourg, France
This study is part of the priority scientific area Brain connectivity and neurodegenerative diseases of the Institut Pasteur's strategic plan for 2019-2023.





