Human evolutionary genetics: the benefits of genetic diversity

The Collège de France recently set up a new permanent chair entitled "Human Genomics and Evolution". It is currently held by Lluis Quintana-Murci, a population geneticist at the Institut Pasteur. He is particularly renowned for his expertise in human genetics because of the medical impact of his basic research – the aim of his work is to identify the genetic basis of immune system variability and resistance to infection. The lectures he gives at the Collège de France demonstrate the enormous potential of population genetics and genomics for understanding diseases. As well as infectious diseases such as malaria, tuberculosis and hepatitis C, population genetics can help explain the development of autoimmune disorders (allergies, inflammation, autoimmunity, etc.) in humans. Put simply, the aim is to understand the evolution of the immune system so as to pave the way for precision medicine. Population genetics also looks at the cultural aspects of human evolution. The multifaceted nature of this research field echoes the wealth of humanity's genetic heritage.
In any given population, individuals differ genetically from one another in a great many of their genes. "Population genetics is a scientific discipline that aims to assess the degree of genetic diversity within a group made up of several individuals," explains Lluis Quintana-Murci, Head of the Human Evolutionary Genetics Unit at the Institut Pasteur. "It looks at the distribution of genes and identifies any changes in their frequency, with the purpose of improving our understanding of the origins of our species, human migrations, and how we have adapted genetically to environmental changes."
Our genetic diversity is governed by several genomic, demographic and evolutionary factors:
- genetic mutations, in other words changes to the DNA sequence of a gene;
- natural selection, a process (proposed by Darwin) by which the natural elimination of the least fit in any given environment enables a population to adapt from one generation to the next;
- genetic drift, the evolution of a population or a species caused by random phenomena that are impossible to predict. The effects of drift are particularly important in small populations;
- genetic migration, the transfer of genes from one population to another because of the migration of individuals.

© Pixabay
Population genetics enables us to study "the origins and age of the human population and the history of the colonization of the planet." The discipline also sheds light on "the impact of our heredity on our health, our predispositions to certain diseases, and even our differential responses to therapeutic treatments," explains Lluis Quintana-Murci, who adds: "Population genetics also investigates how the historical habitat of a population and its lifestyle have influenced our genome... and our epigenome" – those "tags" attached to the genome that do not actually modify DNA itself.
At the Institut Pasteur, population genetics touches on several fields of expertise including human genetics, genomics, evolutionary biology and immunology. The Institut Pasteur's scientists employ a multidisciplinary approach to improve understanding of humans' genetic diversity, especially in relation to infectious diseases. "In Western countries, many genetic studies are carried out on populations of European origin – Europeans and North Americans of European descent –, but we need to open our research up to Africans and Asians." This broader vision of genetic diversity would improve our knowledge of the genetic basis of infectious diseases. "For the past 15 years we have been investigating population genetics in Central Africa (in Cameroon and Gabon), as well as, more recently, Vanuatu and the Solomon Islands in the Pacific," continues Lluis Quintana-Murci. "We also have a project in French Polynesia. And it is important for us to study "neglected populations". It goes without saying that we need to treat all diseases that affect all populations."

© Institut Pasteur
The history of population genetics
Population genetics is a relatively recent discipline. It emerged over the period from the 1920s to the 1940s following research by Ronald Fischer, J.B.S Haldane and Sewall Wright. Population genetics initially applied to populations the fundamental principles of Mendelian genetics, according to which genes are passed on from one generation to the next (named after Gregor Mendel, the Austrian friar and botanist recognized as the founding father of genetics). Experts in population genetics then established a link between Mendelian genetics and the famous theory of evolution developed by Darwin.
In the early 2000s, scientists sequenced the entire human genome, a major breakthrough that improved our understanding of gene location and structure. The chimpanzee genome was also fully sequenced, a key discovery since scientists were able to compare it with the human genome and identify which parts of the human genome are specific to our species.
Several international consortia then met to define polymorphisms, in other words differences between individuals and populations.

©Jérôme Bon
A demographic history, from our origins to the present day
The genetic diversity of the recent human species was particularly shaped by the effects of migration. Humans left Africa around 40,000 to 80,000 years ago. This migration was followed by the rapid colonization of southern Asia, Australia, Europe and eastern Asia. They even went further afield, to the Americas, around 15,000 to 35,000 years ago, then to the remote islands of Oceania, where they settled just 1,000 to 4,000 years ago.
"These migratory routes sometimes give rise to debate," says Lluis Quintana-Murci. "One example is the case of the Bantu peoples, which underwent a wave of expansion some 4,000 to 5,000 years ago, largely because of the emergence of farming." After originating in West-Central Africa, the Bantu population then traveled to the continent's eastern and southern regions.
Question marks had always remained as to the migratory route taken by these peoples:
- a first theory claimed that the Bantu population immediately split into two movements after leaving their homeland, one heading east and one south;
- and a second theory suggested that they crossed the equatorial forest (today part of Gabon) before dividing into two waves of migration, one continuing south and the other heading to East Africa.
A large-scale genomic study involving 2,000 individuals from 57 populations throughout Sub-Saharan Africa enabled scientists from the Institut Pasteur to confirm the second theory.
The scientists' research also demonstrated that during the past millennium, the Bantus admixed with Pygmy populations, and that this admixture was beneficial for the Bantus, conferring advantageous genetic mutations that helped them adapt to their new environments. "Our research pieces together the migratory routes taken by the Bantu peoples and shows that their admixture with local populations was beneficial for their adaptation to the environment, especially in terms of immunity," explains Lluis Quintana-Murci, who coordinated the study. "While we were already familiar with cases showing the acquisition of genetic advantages between species, it is virtually the first time that this concept has been demonstrated within the human population."
Demographic changes can indeed have an impact on natural selection. Population fluctuations and evolutionary history have resulted in mutations that are both beneficial and harmful for human health.
So studying genome diversity is vital if we are to identify which of these mutations contribute to susceptibility to complex illnesses, such as infectious or autoimmune diseases.

"Square" houses in dry mud, Bongo Pygmies, Gabon, 2006. © Paul VERDU / MNHN / CNRS Photo library
How our culture has influenced our genetic heritage
The emergence of agriculture, which began in different regions of the world over the past 10,000 years, proved to be a complete revolution for the human species in technological, cultural and environmental terms.
In his book Genes, Peoples and Languages, Luca Cavalli-Sforza explains that "it would be inexcusably superficial to investigate human evolution without paying sufficient attention to the cultural aspects."
Population genetics can be used to examine the links between culture and nature more closely. Recent research by scientists in the Institut Pasteur's Human Evolutionary Genetics Unit has challenged long-held beliefs that the emergence of agriculture led to significant population growth.
While the development of agriculture has been pinpointed to approximately 5,000 years ago, the genomic study carried out by the Institut Pasteur's scientists revealed that the population explosion dates from well before this period (7,000 to 10,000 years ago). "The ancestors of the peoples we refer to as the Bantus, who were hunter-gatherers, experienced such massive demographic growth that they needed to turn to a new way of life to survive," explains Lluis Quintana-Murci, Head of the Human Evolutionary Genetics Unit at the Institut Pasteur.
But how is it possible for us to adapt to a new environment? How were these humans able to change their habitat (forest, rural or urban environment) and lifestyle (nomadic hunter-gatherer or sedentary farmer)?
It is actually the environment that exerts selective genetic pressure and can bring about changes in the frequency of some DNA mutations that are advantageous for our species. And that's not all: the Institut Pasteur's scientists also discovered that the environment influences the epigenome.
By comparing different populations from Central Africa, they observed that the change in habitat (from forest regions to more rural and even urban environments) altered the epigenome, especially functions affecting the immune system.
"We need to conduct further research to investigate how epigenetic changes can lead to an immune system that is more prone to developing autoimmune disorders, allergies, inflammation and so on," explains Lluis Quintana-Murci.

Pygmy women and children opening the mangoes to extract the almonds and recover the pulp, near the “snake camp”, east of the city of Lomié in Cameroon. This photograph was taken during the follow-up of the ANR GrowinAP (2011 -2015): "Diversity of human growth. Growth among African Pygmies". © Laurent MAGET / EAE / CNRS Photo library
Our health today – a legacy of evolution
To shed light on our predispositions to some diseases, scientists turned to genetics resources. In the late 2000s, the HapMap project was launched to develop a public resource based on genetic data that could help scientists discover the genes associated with human diseases and drug response. The HapMap ("haplotype map"), accessible free of charge anywhere in the world, represents a crucial resource for scientists.
In 2008, scientists from the Institut Pasteur used these data to identify hundreds of genes that vary between human populations. Some of these genes are involved in human disease, like the CR1 gene, which influences the severity of malaria cases. The scientists demonstrated that 85% of Africans carry a mutation in this gene, but that the mutation is missing from European and Asian populations. "By identifying candidate genes for predisposition to certain diseases, our work paves the way for genetic research with medical applications," explains Lluis Quintana-Murci.
Malaria is a particularly interesting example when it comes to the influence of natural selection on the genome and the way in which lifestyle and means of subsistence have affected our demographic history, and especially our susceptibility to some diseases.
Hemoglobin is a molecule in red blood cells that transports iron. In its S form, which is the result of a mutation, it induces sickle cell disease (a blood disorder which leads to health problems including chronic anemia) in homozygous individuals (who have two identical versions of the gene). But when hemoglobin S is present in its heterozygous state (two different versions of the gene), this mutation confers significant protection against malaria.
"The fact that this mutation, while harmful, continues to exist in the population shows that it must give heterozygous individuals a major advantage," explains Lluis Quintana-Murci.
Mathematical models in population genetics can now be used to date the emergence of mutations. "Our theory was that if we could date the emergence of this mutation, which represents an advantage in fighting malaria, it would enable us to date the emergence of malaria in Africa," continues Lluis Quintana-Murci.
The findings show that malaria arrived at least 25,000 years ago in Africa among the ancestors of the Bantus, and that it spread to Pygmies approximately 7,000 years ago according to a theory of "adaptive" admixture. This is an example of the "poison-antidote" theory: the disease was transmitted along with a means of tackling it. The Bantus, a hunter-gatherer people based in savanna regions, suffered from malaria. When they emigrated to the forest, the Bantu populations transmitted the disease to the environment of the Pygmies. But their admixture brought the Pygmies a mutation which, in its heterozygous form, also equipped them to resist it.
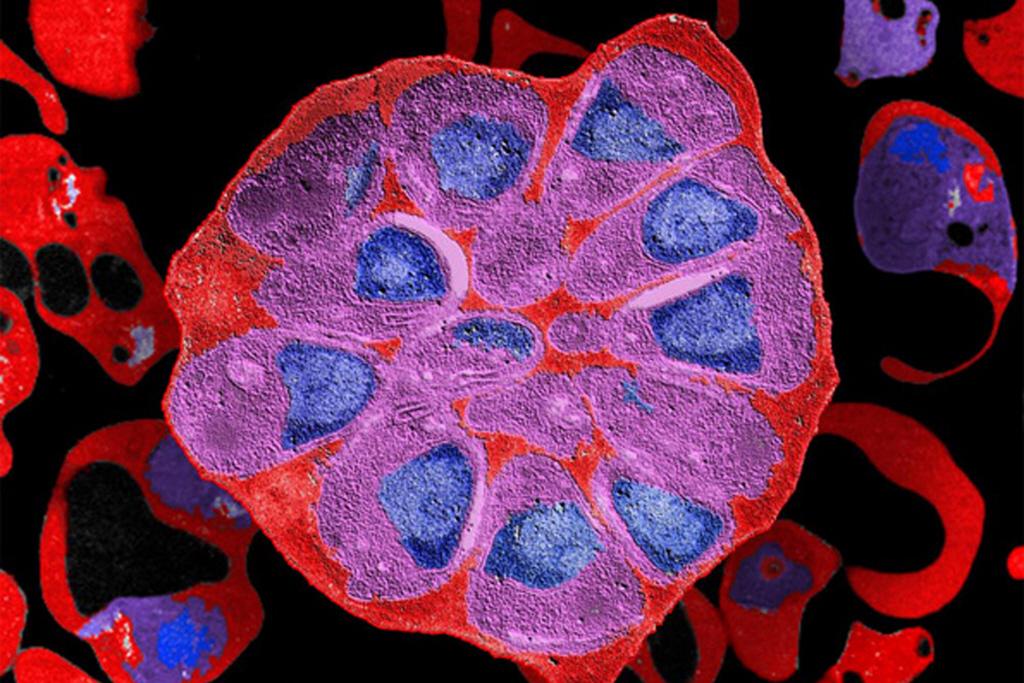
Red blood cell, parasitized by Plasmodium falciparum. © Institut Pasteur
Population genetics: working towards precision medicine
Modern humans are the result of their history: their demographic history, lifestyle and past culture, the pathogens they have come into contact with and the selective measures taken to resist them, and also their admixture history.
Today's scientists are keen to move towards a medical approach based on a significant number of factors that influence patients' response to treatment and susceptibility to disease. To this end, it is important to understand how our current genetics and epigenetics, together with other factors such as our age, sex, diet and microbiota, affect our immune responses.
Recent research at the Institut Pasteur, for example, in collaboration with the "Milieu Intérieur" consortium (see inset below), demonstrated that smoking, age, sex and latent infection with cytomegalovirus were the main non-genetic factors affecting variations in the parameters of human immune cells.
The scientists also showed that the parameters of innate cells are more strongly regulated by genetic variations, whereas those of adaptive cells are mainly modified through exposure to the environment.
The scientists then set out to understand the immune profiles induced by bacteria, fungi and viruses in an age- and sex-balanced cohort of 1,000 healthy individuals. "We observed that age and sex affected the transcriptional response of most genes associated with the immune system. The effects of age were more stimulus-specific than the effects associated with sex," explains Lluis Quintana-Murci. The scientists also demonstrated that specific cell populations were linked with the impact of age and sex on gene expression – CD8+ T cells for age and CD4+ T cells and monocytes for sex. Their research clearly demonstrates cell heterogeneity in the interindividual variability of immune responses, providing valuable information for a more thorough investigation of the risks of infection and disease progression.
Although remarkable progress has been made in our understanding of our history, humanity and biological systems, some mysteries remain.
Polynesia is a source of major interest in evolutionary biology, since it is the last place in the world to have been populated by humans, around 900 to 1,000 years ago.
"Some key public health issues have been identified in Polynesia. For example, the population has to deal with two influenza outbreaks each year – a specific Polynesian influenza and a European influenza introduced by travelers. Moreover, Polynesian populations also suffer from a number of metabolic problems such as high blood pressure, diabetes and obesity. I hope that our future research will offer some answers so that real improvements can be made to their health."
In 2018, Lluis Quintana-Murci's team received funding from the French Foundation for Medical Research (FRM) to carry out further research on influenza infection. "By using single-cell sequencing techniques to shed light on how age and sex influence host transcriptional response to influenza, we hope to identify new avenues of research that will pave the way for a better understanding of the disease."
The Milieu Intérieur project
Predisposition to infection, the severity of disease and the response to drugs and vaccines all vary significantly from one individual to the next. The complexity of immune responses in individuals and populations previously prevented scientists from identifying the parameters – both genetic and environmental – that create a healthy immune system and are responsible for its natural variability. The Milieu Intérieur consortium, hosted at the Institut Pasteur, is attempting to establish the parameters that characterize the immune system of a healthy person by drawing on resources from several disciplines (immunology, genomics, molecular biology and bioinformatics). This is a vital stage in efforts to develop personalized medicine, in other words applying a specific therapeutic strategy to a given person at a given time.

The principles of the Milieu Intérieur project
To examine the genetic and environmental factors that influence the variability of immune responses, the Milieu Intérieur project has assembled a cohort of 1,000 healthy people. The project will have a huge impact for public health, since the data can help establish benchmark values which can be used to define disrupted immune responses in individuals. Several pilot studies are already under way for various diseases, including tuberculosis, hepatitis C and type 1 diabetes.
The Milieu Intérieur project was selected following a call for proposals launched by the "Laboratories of Excellence" program, which encourages the emergence of ambitious scientific projects at international level.
Lluis Quintana-Murci, a researcher specializing in "humanity"
Lluis Quintana-Murci was born in 1970 in Palma de Mallorca, Spain.
He arrived at the Institut Pasteur in Paris in 1999, joining the Human Immunogenetics Unit led by Marc Fellous. That same year, he published his first findings on the routes taken by humans when they left Africa more than 60,000 years ago. In 2005, he obtained his accreditation to supervise research, giving him the opportunity to open his own unit, Human Evolutionary Genetics, in 2007.
Lluis already has more than 200 scientific articles to his name. His extensive research on genome diversity helps us understand how our species is able to adapt to its environment. When humans migrated from Africa to other world regions, they underwent mutations over thousands of years to adapt to climatic differences, the nutritional resources available to them and the pathogens they came into contact with.

Lluis Quintana-Murci,
Head of the Human Evolutionary Genetics Unit at the Institut Pasteur.
© College de France
In France, as soon as we talk about diversity, we are scared. While we should make this diversity an allegory of our differences!
To find out more, read our profile "Lluis Quintana-Murci: Discovering the world and humankind"
Population genetics comes to the Collège de France
A new permanent chair "Human Genomics and Evolution" has been created at the Collège de France. Interview with Lluis Quintana-Murci, a population geneticist at the Institut Pasteur (Paris), who holds it. Video copyright: Institut Pasteur
Learn more about the inaugural lesson on February 6, 2020, 6 p.m. (page in French)
In 2019, Lluis Quintana-Murci was appointed as holder of the permanent chair in "Human Genomics and Evolution" at the prestigious Collège de France. The aim of the lectures he gives as holder of this new chair is to demonstrate how progress in knowledge on the variability of the genome among human populations and the different factors that shape this variability is helping us to understand the demographic history of humans, how they have adapted to their environment, and the links between genetic diversity and phenotypic diversity, whether harmless or responsible for disease. Topics that will be examined include:
- an introduction to population genetics,
- genetic and phenotypic diversity in humans,
- a genetic reconstruction of the demographic history of our species,
- natural selection and adaptive phenotypes,
- genetic diversity and cultural forces,
- human adaptation to pathogens: immunity and infectious diseases.
These courses given at the Collège de France are free, open to everyone, without registration conditions (subject to availability).





