Pathogenic bacteria produce a number of molecules that can be recognized by the innate immune system. These molecules associated with pathogens trigger an inflammatory response by stimulating specific signaling pathways in infected immune cells. Scientists from the Institut Pasteur and the CNRS, in cooperation with the University of Massachusetts Medical School, have demonstrated that group B Streptococcus degrades one of these molecules so that it can control the inflammatory response of the infected host. These findings, published in Cell Host and Microbe on July 13, 2016, help improve our understanding of the interaction between bacteria and immune cells during the infectious process.
The innate immune system is our first line of defense during microbial infection. The initial stages of this immune response involve the recognition of specific molecules associated with these microbes. This information is then used by cells to provide a coordinated response tailored to the invading microbes so that the threat can be eliminated. However, some pathogens have developed defense mechanisms to avoid being recognized by the immune system.
Type I inferferons are one of the molecules produced by the immune system in response to microbial infection. Although better known for their role in viral infections, type I interferons are also needed to fight infections caused by some bacterial species, such as group B Streptococcus (GBS), a bacterium responsible for invasive infections in newborns.
Scientists from the Biology of Gram-Positive Pathogens Unit, directed by Patrick Trieu-Cuot (Institut Pasteur/CNRS), working together with scientists from the University of Massachusetts, discovered a new mechanism that enables bacteria to limit interferon production by infected immune cells. The production of interferon following GBS infection mainly depends on the cells recognizing two types of molecule released by the bacteria: bacterial DNA and cyclic di-AMP, a specific signaling nucleotide which controls the activity of many of the bacteria's vital functions. The scientists characterized an enzyme at the surface of GBS, known as CDNP, which hydrolyzes its own cyclic di-AMP that is released in the cytoplasm of immune cells. CDNP thereby controls interferon production and boosts the pathogen's virulence.

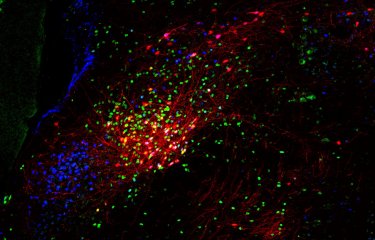
Macrophages infected by group B Streptococcus. The sample was observed using fluorescence microscopy. The actin filaments in the macrophage are shown in green and the bacteria in red. © Institut Pasteur / E. Davenas and P. Trieu-Cuot.
This original mechanism, in which bacteria degrade their own molecules to avoid being recognized by the immune system, may also exist in other pathogens. Blocking this camouflage mechanism using new antimicrobial drugs would enable the immune system to recognize and eliminate invasive bacteria more quickly.
This research is supported by the Institut Pasteur and the CNRS; it has also received funding from the FRM and the IBEEID Labex.
Source
Group B Streptococcus Degrades Cyclic-di-AMP to Modulate STING-Dependent Type I Interferon Production, Cell Host and Microbe, July 13, 2016
Warrison A. Andrade,1,2,6 Arnaud Firon,3,4,6 Tobias Schmidt,5 Veit Hornung,5 Katherine A. Fitzgerald,1,2 Evelyn A. Kurt-Jones,1,2 Patrick Trieu-Cuot,3,4 Douglas T. Golenbock,1,2,7 and Pierre-Alexandre Kaminski,3,4,7
1Division of Infectious Diseases and Immunology
2Program in Innate Immunity Department of Medicine, University of Massachusetts Medical School, Worcester, MA 01605, USA
3Institut Pasteur, Unité de Biologie des Bactéries Pathogènes à Gram-Positif, 75724 Paris, France
4Centre National de la Recherche Scientifique (CNRS) ERL 3526, 75724 Paris, France
5Institute of Molecular Medicine, Universitätsklinikum Bonn, Bonn 53127, Germany
6Co-premier auteur
7Co-senior auteur
Mis à jour le 13/07/2016