Nanotubes have been observed for the first time in a living organism.(1) This discovery, made by an Institut Pasteur laboratory, is the culmination of twenty years of research on these intriguing intercellular connections.
"I've believed in tunneling nanotubes since the beginning!" says Chiara Zurzolo, Head of the Membrane Traffic and Pathogenesis Unit at the Institut Pasteur. She has been researching nanotubes for the past twenty years. Originally trained in medicine to gain a solid foundation for cellular biology, she joined the Institut Pasteur in 2003. At that time, she witnessed the early discoveries surrounding nanotubes, when a team from Germany and Switzerland(2) first observed, in vitro, that cells could communicate through narrow channels—tiny tunnels known as tunneling nanotubes (TNTs). Cells use these structures to transfer various substances, including hormones, enzymes, organelles, and vesicles.
Initially, the discovery was met with skepticism, as researchers debated whether these structures played a significant role in cellular communication. But for Chiara Zurzolo, TNTs represented a fascinating and promising field of study, and she dedicated her career to investigating them. Twenty years later, in 2024, she and her team have made a breakthrough: for the first time, they have observed TNTs in a living organism, confirming their existence in vivo.*
Nanotubes observed in vivo by fluorescence
The Institut Pasteur's Membrane Traffic and Pathogenesis Unit is a team of around 15 early-career scientists from all over the world. For this project, which aimed to provide evidence of TNTs in vivo, they chose the zebrafish due to its transparent body, which makes it ideal for observation under a microscope.To investigate whether TNTs play a crucial role in zebrafish development, they fluorescently labeled embryo cells.
They discovered that most of the protrusions extending from these cells were long, hollow tubes—nanotubes! Using live-cell imaging, the team also demonstrated that mitochondria, the cell's "powerhouse," can transfer between cells via TNTs. This study marks the first time that TNT behavior, previously observed only in vitro, has been confirmed in a living organism. "Our data also suggests that TNTs play a part in the healthy development of zebrafish," emphasizes Chiara Zurzolo.
Structure and precise role of TNTs yet to be elucidated
Much about nanotunnels remains unknown, including their exact structure and precise molecular composition. Another key area of research is their role in disease. While TNTs can transport beneficial substances essential for cellular function, they can also serve as conduits for harmful agents.

Pioneering research by Chiara Zurzolo's team has shown that TNTs facilitate the spread of several pathogenic proteins. This includes prions and amyloid proteind (like alpha-synuclein and Tau) linked to neurodegenerative diseases such as Creutzfeldt-Jakob disease, Parkinson's, and Alzheimer's. TNTs have also been implicated in the spread of viruses like SARS-CoV-2, which infects the olfactory bulb and may contribute to anosmia and neurological symptoms. Additionally, TNTs may play a role in cancer by transferring tumor-promoting molecules.
Recent research on nanotubes at the Institut Pasteur
|
TNTs involved in the spread of neurodegenerative diseases and the proliferation of cancer cells
"TNT-mediated protein transfer is a key mechanism in the progression of neurodegenerative diseases in the brain," confirms Chiara Zurzolo. TNTs are also believed to contribute to brain cancer. "By allowing mitochondria to move freely between cells, they may enhance or even accelerate cancer cell division," explains Zurzolo.
For all these reasons, tunneling nanotubes represent major therapeutic targets. Targeting specific molecules within the TNT structure could allow scientists to inhibit or eliminate these nanotubes, potentially slowing disease progression or even preventing its onset. However, before TNTs can be targeted therapeutically, researchers must first unravel their relationship with inflammation—an essential factor in many diseases that appears to exploit TNTs to promote its own spread.
Nanotubes: a novel conception of cellular identity
BEFORE: Cells were once thought to be isolated, "closed" entities.
Cells could only communicate with each other through secreted molecules, such as enzymes or hormones. Below, in this highly simplified drawing, the arrow indicates the secretion and transfer of molecules or vesicles from one cell to another. The cell membrane (black circle line) is the interface between the cell interior (cytoplasm) and its external environment.
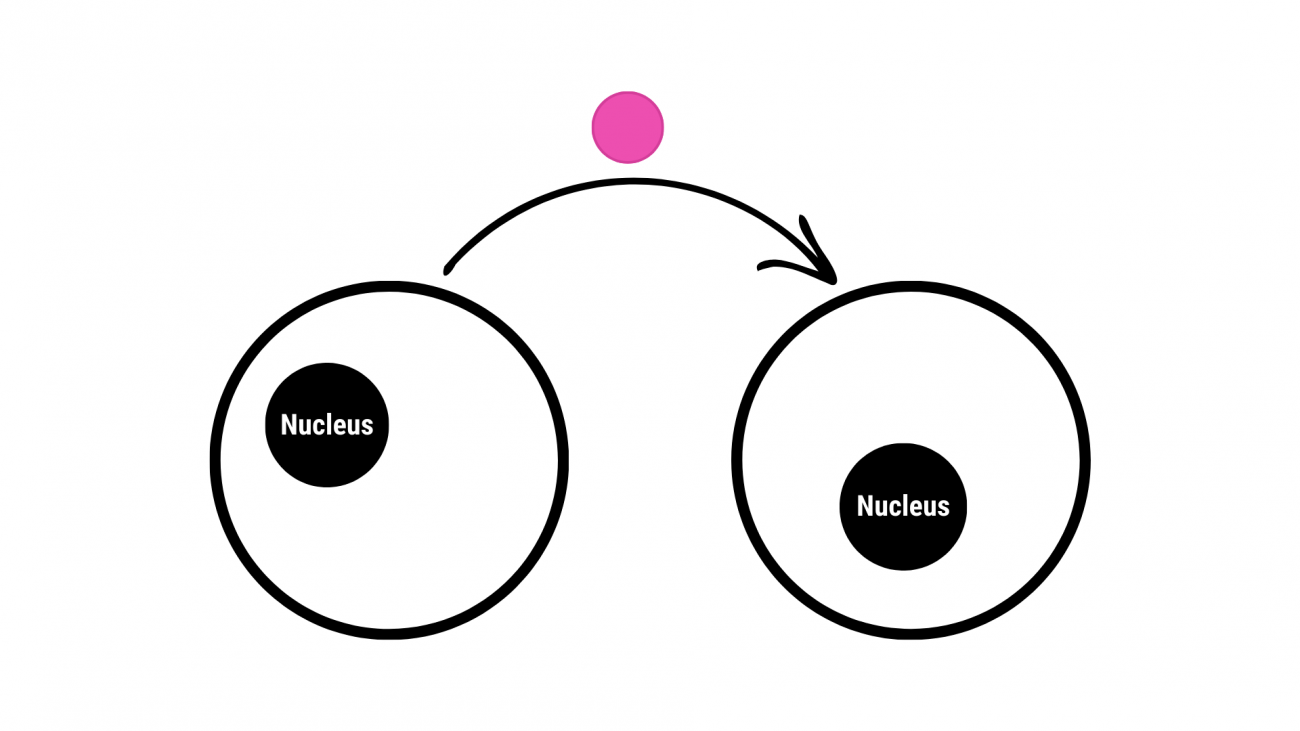
AFTER: These reecent discoveries have shown that cells are not isolated entities.
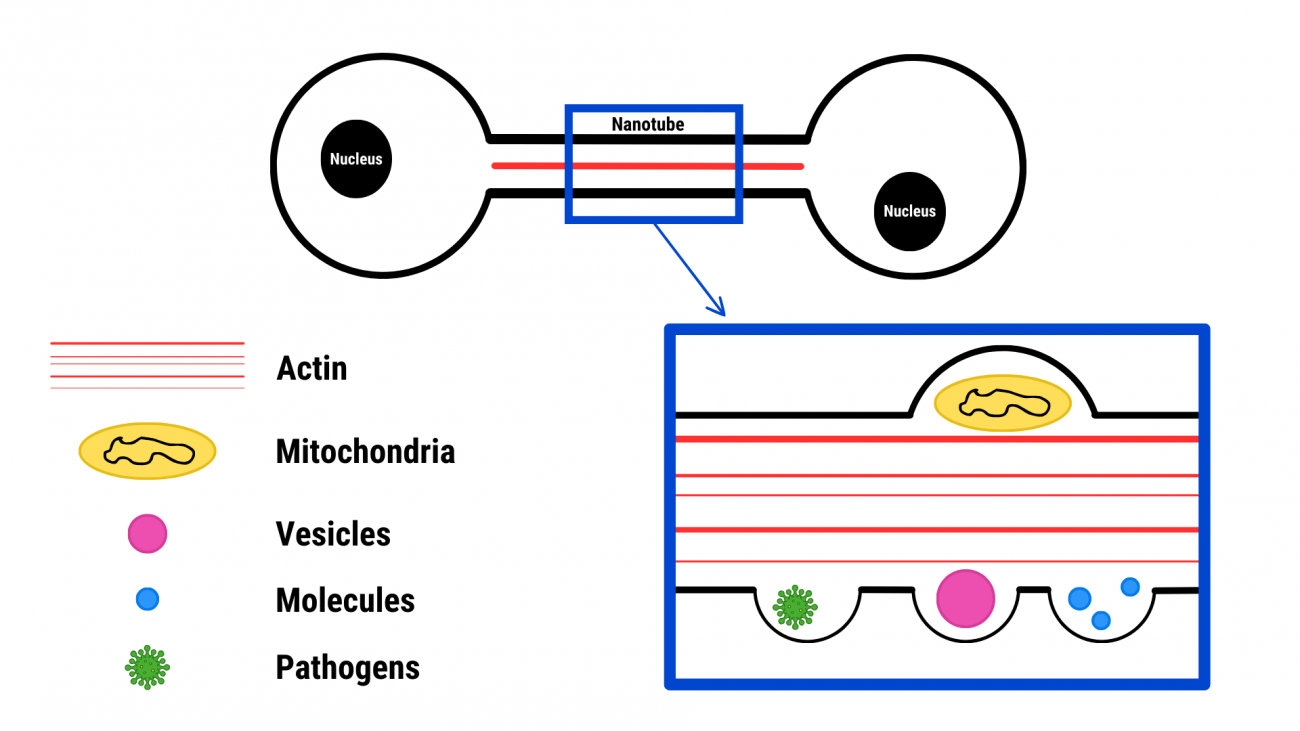
Beyond secretion-based transfer, cells can also share their internal contents directly with other cells. A dedicated transfer channel known as a nanotube enables them to communicate in a direct and highly dynamic way. While this research confirms the presence of TNTs in living organisms, the precise mechanisms governing their formation and molecular exchange remain unclear. Understanding these processes is crucial before TNTs can be explored as therapeutic targets. This groundbreaking research underscores the power of fundamental science in driving revolutionary discoveries—reshaping our understanding of biology and disease pathophysiology. Below: a simplified drawing of these TNT nanotubes and what they carry from cell to cell.
The microscopic revolution to see nanotubesRecent developments in microscopy techniques are providing us with increasingly precise images, facilitating the visualization of nanotubes.
As well as microscopy, organoids can be engineered to form 3D organ cultures in vitro, enabling scientists to study highly precise miniature versions of the brain, for example. |
- Tunneling nanotubes enable intercellular transfer in zebrafish embryos, Korenkova O, Liu S, et al., Developmental Cell, 2025
- Nanotubular highways for intercellular organelle transport. Rustom A, Saffrich R, el al., Science, 2004





