Cancer is the leading cause of mortality in France. In the last 40 years, survival rates have increased for several types of cancer, such as breast or prostate cancer. However, brain cancer is still an incurable disease, as it usually becomes resistant to therapy and relapses in a relatively short time. The outcome is almost inexorably the patient death. Researchers headed by Chiara Zurzolo at the Institut Pasteur have studied tunnelling nanotubes in glioblastoma, the most aggressive brain cancer.
Glioblastoma (GBM) is the most aggressive, invasive and lethal of the central nervous system tumors. The incidence of GBM is of 3 people every 100.000. This form of cancer is incurable, median survival being 14.6 month. Despite the current treatments, combining surgery followed by concomitant radio and chemotherapy, GBM relapse is almost inevitable. Tumor relapse arises in most cases from Glioblastoma Stem Cell population (GSCs).
Glioblastoma cancer cells communicate through tunneling nanotubes and tumor microtubes
How cells communicate in invasive cancers has become promising avenue of therapeutic research. Recently, increasing interest has been given to a new type of intercellular communication named Tunneling Nanotubes (TNTs). TNTs are thin, open channels connecting distant cells and allowing long distance communication between cells. TNTs presence in different cancers was associated with a more malignant tumoral phenotype. TNTs can also be present in several normal cells and can be hijacked by various pathogens, such as bacteria, viruses and prions. Glioblastoma were recently found to form a multicellular network propagating electrical signals through cell extensions named Tumor Microtubes (TMs), similar to TNTs, but unable to transfer cellular material. Interestingly, the extent of this network was correlated with high resistance to treatment.
TNTs connection between GSC might be linked to tumor resistance and progression
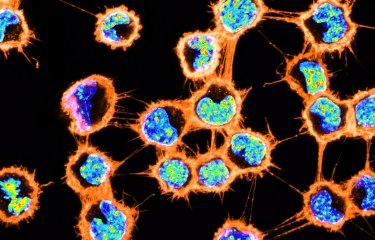
In order to investigate these mechanisms, researchers used Glioblastoma Stem Cells (GSCs) derived from different patients and from different zones of the tumor. Using fluorescent microscopy, in addition to TMs, they observed TNTs connections between GSCs grown in 3D tumor organoids. They also demonstrated their capacity to transport large cargoes like mitochondria. Mitochondria are double-membrane bound organelles that control the life and death of the cell as they represent their powerhouse. The ability to share this energy source can therefore provide a pro-tumoral thrust in various cancer forms. The researchers observed that TNT-based communication was highly resistant to radiotherapy. Thus, TNT presence could be advantageous for cancer cells making them more aggressive or more apt to survive to the cellular stress induced with the administration of therapies.

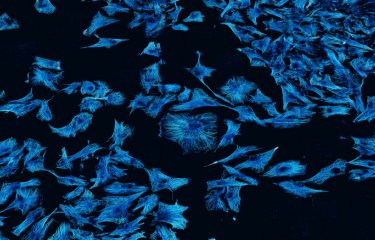
TNT-like structures exist in GSLC tumor organoids together with TM-like protrusions
Tumor organoids are a relevant 3-dimensional, culture method which allows long-term growth preserving the stem cell identity of cells and reconstituting to some extent the morphological and phenotypic heterogeneity of the original tumor. Representative fluorescence image of a tumor organoid from GSCs after 23 days of culture, stained for anti-αTubulin (microtubules, white), Phalloidin (actin red). Different types of connections are present and coexist inside the organoid. Copyright: Institut Pasteur/Chiara Zurzolo
This data shows for the first time in patient derived tumor organoids, that TNTs participate to GBM tumoral networking allowing cargo transfer. This might promote tumor progression and therapy-resistance. Furthermore, as TNT-based communication can be impaired by drug treatments, TNTs could be an important therapeutic target.
This work was carried out thanks to the bequest made by Mrs Marguerite Michel.
Source
Patient-derived glioblastoma stem cells transfer mitochondria through tunneling nanotubes in tumor organoids, biochemical journal, 8 January 2021
Giulia Pinto1,2,*, Inés Saenz-de-Santa-Maria1,*, Patricia Chastagner1, Emeline Perthame3, Caroline Delmas4, Christine Toulas4, Elizabeth Moyal-Jonathan-Cohen4, Christel Brou1,* and Chiara Zurzolo1,*
1Unité de Trafic Membranaire et Pathogenèse, Institut Pasteur, UMR3691 CNRS, 28 rue du Docteur Roux, F-75015 Paris, France;
2Sorbonne Université, ED394 — Physiologie, Physiopathologie et Thérapeutique, F-75005 Paris, France;
3Hub de Bioinformatique et Biostatistique — Département Biologie Computationnelle, Institut Pasteur, USR 3756 CNRS, F-75015 Paris, France;
4Cancer Research Center of Toulouse (CRCT), Institut National de la Santé et de la Recherche Médicale (INSERM), UMR 1037, Institut Claudius Regaud, Université Toulouse III Paul Sabatier, Toulouse F-31000, France
*These authors contributed equally to this work.
This study is part of the Cancer Initiative of the Institut Pasteur's strategic plan for 2019-2023.