Researchers at the Institut Pasteur and CNRS have just determined the structure of a bacterial protein similar to the human nicotine receptor, and have published this result in the journal Nature. This is an important step for the molecular modeling of substances able to interact with this receptor and which could help treatment of nicotine addiction.
Nicotine is the principal substance in tobacco causing addiction, particularly in terms of the impairment it causes to the reward system, which is the natural management system of our desires, pleasures and emotions. At this level, it acts by interacting with receptors known as nicotinic.
Press release
Paris, november 5, 2008
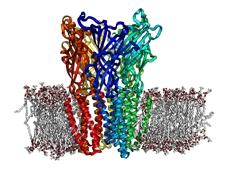
“This bacterial homolog has been discovered by comparative genomics. It is derived from a common ancestor dating back between one and three billion years,” explained Pierre-Jean Corringer. “Faced with the difficulty of obtaining a 3D structure of the human nicotinic receptor, we chose to establish a 3D structure of this bacterial protein, which does not have exactly the same function, but is similar to the human molecule from a purely structural point of view.”
The 3D structure of another bacterial homolog of the human nicotinic receptor had already been obtained at the beginning of 2008 by a different team, but this was a structure in the “inactive” state, whereas the Pasteur teams established the structure of the protein in an “active” state, in other words a true physiological state. In this way they were able to compare the two structures in order to understand how these receptors are activated by pharmaceutical compounds.
This will enable researchers to draw closer to the 3D structure of the human nicotinic receptor and also to advance in the field of molecular modeling, or drug design: it is a matter of finding molecules able to interact with the nicotinic receptor, in other words candidate nicotine withdrawal drugs.
We should not forget that, according to the World Health Organization, tobacco is the second cause of mortality throughout the world, and is currently responsible for one in six adult deaths.
In addition, the bacterial receptors studied by researchers are also homologous to other human receptors, in particular GABAA receptors, which are the targets of general anesthetics and anxiolitics. The 3D structure obtained by the work of researchers at the Institut Pasteur could therefore have an impact on the design of these compounds.
_________________
1. URA CNRS 2182
2. URA CNRS 2185
Sources
“X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation”: Nature, advanced publication online, November 5th, 2008
Nicolas Bocquet (1)*, Hugues Nury (1,2)*, Marc Baaden (4), Chantal Le Poupon (1), Jean-Pierre Changeux (3), Marc Delarue (2) & Pierre-Jean Corringer (1)
1. Institut Pasteur, Channel-Receptors 5-year group, CNRS URA 2182, Paris
2. Institut Pasteur, Structural Dynamics of Macromolecules Unit, CNRS URA 2185, Paris
3. Institut Pasteur, CNRS URA 2182, F75015, Paris
4. Institute of Physical Chemical Biology, CNRS UPR 9080, Paris, France.
Contact persons
Institut Pasteur Press Office: Corinne Jamma or Nadine Peyrolo - +33 (0)1 40 61 33 41 - cjamma@pasteur.fr
CNRS Press Office - +33 (0)1 44 96 51 51 - presse@cnrs-dir.fr