Introduction
People with hearing loss are deprived of the social interactions linked to speech perception. This can lead to psychological distress due to feelings of isolation, and often to depression. Social bonds are jeopardized, resulting in a gradual loss of independence. Age-related hearing loss, which develops from the age of about 50, has recently been identified as the leading risk factor for dementia.
However, new hopes have recently been raised by groundbreaking studies, in the Institut Pasteur Genetics & Physiology of Hearing Unit, in particular, and by the opening of the Hearing Institute, a new dedicated Institut Pasteur research center created at the initiative of the Fondation Pour l'Audition. These recent breakthroughs pave the way for:
- The extension of research to late-onset forms of hearing loss, central hearing loss and hearing loss induced by noise, the main environmental threat to the auditory system;
- The development of new medical approaches for hearing loss, combining innovative diagnostic approaches and curative treatments (targeting the cochlea and the neurons innervating the auditory system).
The Hearing Institute will bring together experts in areas as diverse as biophysics and cognitive neuroscience, creating the ideal conditions for major improvements in our understanding of the development and workings of the auditory system as a whole, through studies at multiple scales, extending from molecules to behavior. Translational research at the Hearing Institute will be facilitated by high-level expertise in basic auditory neuroscience; a center for research and innovation in human audiology (CERIAH); and partnerships with pediatric and adult ENT departments from the university hospitals in France (in Clermont-Ferrand and Lyon in particular) and the Parisian public hospital network (AP-HP), with ENT specialists in the private sector and with other hearing health professionals, including hearing aid specialists.
Causes of hearing loss
Hearing loss can take many forms, which can be classified according to:
- The degree of hearing loss – mild, moderate, severe or profound;
- Whether or not the hearing loss is associated with other clinical signs – nonsyndromic or syndromic hearing loss;
- The principal site of auditory system impairment – conductive (mechanical) hearing loss, associated with a problem in the outer or middle ear, and sensorineural (perceptive) hearing loss, caused by a problem somewhere within the auditory system, from the cochlea to the auditory cerebral cortex.
Hearing loss may be broadly attributed to genetic or environmental factors. In developed countries, severe or profound hearing loss diagnosed at birth or before language acquisition is mostly caused by genetic mutations. These forms of hearing loss are generally “monogenic”, which means that they can be attributed to a single gene. Genetic factors also contribute to age-related hearing loss (also known as presbycusis), but the precise causes of this condition remain unknown.
The environmental factors underlying hearing loss include infections (prenatal infection with cytomegalovirus (CMV), viral or bacterial otitis, etc.) and iatrogenic causes (the use of ototoxic drugs, which are toxic specifically to the ear, including some antibiotics (such as aminoglycosides) and chemotherapy treatments (cisplatin)).
The main environmental threat to the auditory system, at all ages and worldwide, is overexposure to noise. The auditory system is, thus, the most sensitive of all the sensory systems to the environmental changes associated with urbanization.
For each of these environmental factors, there are genetic susceptibility factors accounting for the difference in hearing impairment between individuals, even if they have been exposed to the same environmental agents. According to the World Health Organization (WHO), 1.1 billion young people are currently at risk of hearing loss, mostly because they listen to loud music for long periods of time through headphones.
Conductive hearing loss, which often occurs after an ear infection, is generally mild or moderate. This form of hearing loss may be more frequent in individuals with ear malformations (such as aplasia) associated with a hereditary condition. Otosclerosis, which leads to conductive hearing loss in young adults at about the age of 30, is caused by a problem with the stapes, a bone in the middle ear. It has been linked to both genetic and environmental causes.
Sensorineural or perceptive hearing loss is much more common than conductive hearing loss. It is mostly caused by damage to the cochlea, and the degree of hearing loss is variable. Severe or profound sensorineural hearing loss (SNHL) can generally be attributed to genetic mutations. SNHL may also be caused by acoustic trauma or may occur suddenly for no obvious reason – a phenomenon known as sudden sensorineural hearing loss (SSHL). In France, SNHL affects one infant in 700 at birth and approximately one child or young adult in 500 before the age of 20. It leads to major difficulties in spoken language acquisition and school-based learning. Treatment for children with hearing loss involves educational support, speech therapy and the fitting of a hearing aid or cochlear implant.
Presbycusis, or age-related sensorineural hearing loss, accounts for about 90% of all cases of hearing loss. It can be attributed to both genetic and environmental causes, primarily overexposure to noise. According to current estimates, presbycusis affects one third of people over the age of 65. The number of people with presbycusis is continually rising, due to both the aging of the population and noise overexposure as a result of the ever-increasing density of city populations.
Hearing loss associated with overexposure to continuous noise or to impulse noise (almost instantaneous sharp noises, such as clicks and pops) is the leading source of military health spending in the United States and the second leading source of such expenditure in France.
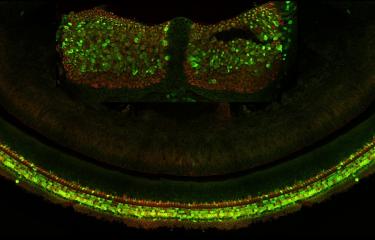
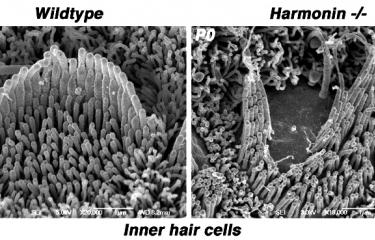
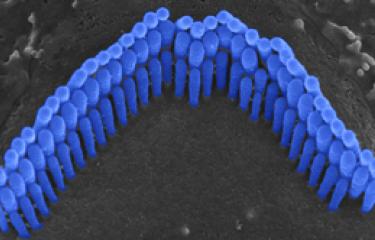
Over the last 25 years, sensorineural hearing loss in young people has been classified into more than a hundred types, each defined on the basis of damage to a specific gene. Genetic research into hereditary forms of hearing loss in humans has also effectively elucidated the molecular physiology of the cochlea. A particular example of this is provided by Usher syndrome, a highly debilitating sensory condition affecting hearing, sight, and sometimes also balance. By identifying the genes responsible for the most severe form and analyzing the hearing impairments associated with defects of these genes, the scientists in the Genetics & Physiology of Hearing Laboratory at Institut Pasteur were able to determine the composition of the molecular machinery responsible for converting sound energy into electrical signals. This discovery of the genes involved in Usher syndrome has also led to new molecular diagnosis techniques, making it possible to diagnose this condition in infants well before the onset of vision loss. As a result, treatment with cochlear implants can be advanced to much earlier ages.
Epidemiology
According to the World Health Organization (WHO), 466 million people worldwide, including 34 million children, currently have disabling hearing loss. Experts believe that, by 2050, more than 900 million individuals, or one person in 10, will have disabling hearing loss.
Treating hearing loss
Conductive hearing loss can generally be treated by antibiotics or surgery, but this is not the case for sensorineural hearing loss. Hearing devices are the only treatment currently available for sensineural hearing loss. Depending on the severity and location of the impairment, these devices may take the form of:
- Conventional hearing aids;
- Or cochlear implants.
Conventional hearing aids are sound amplifiers. The use of digital sound processing has considerably improved the efficacy of these devices, which are designed for use by people with moderate or severe hearing loss. Cochlear implants make use of an electro-acoustic system that bypasses sensory cells and stimulates the auditory nerve neurons directly. They are generally used in children and profoundly deaf adults, provided that the auditory nerve neurons and the neurons responsible for carrying and processing sound information are intact. Sounds are picked up by an external microphone and transmitted to an external microprocessor, which converts them into electrical signals. These electrical signals are then transmitted to electrodes, each of which stimulates a group of neurons in the auditory nerve. Cochlear implants have proved highly successful in young children, because this efficacy is highly dependent on brain plasticity, which allows the reorganization of neural networks in the auditory cortex according to the sound-encoding electrical signals received. Adults with profound prelingual (onset before language acquisition) hearing loss no longer have the same level of brain plasticity and cochlear implants are therefore much less effective and their use requires a lengthy period of rehabilitation with speech therapists. In adults with profound postlingual (onset after language acquisition) deafness, the auditory brain developed before the onset of deafness and can therefore adapt to the new electrical signals provided by the cochlear implant. However, even with a cochlear implant, the appreciation of complex sounds, such as music, remains unsatisfactory.
As an illustration of the limitations of cochlear implants in particular types of hearing impairment, Institut Pasteur scientists have shown that, in a specific form of hearing loss, DFNB59 auditory neuropathy, caused by a deficiency of the pejvakin protein, the auditory nerve neurons are hypervulnerable to any acoustic or electrical stimulation. As a result, the electrical stimulation produced by the cochlear implant may not have the desired effect.
The first gene therapy clinical trial in France, called Audiogene, is currently underway. Audiogene was developed by a French consortium composed of teams from the Hearing Institute (UMR Institut Pasteur, Inserm, CNRS / IHU reConnect); the ENT Department and Pediatric Audiology Research Center at Necker-Enfants Malades Hospital (AP-HP); Sensorion and Fondation Pour l'Audition. The trial targets the OTOF gene, discovered in 1999 at the Institut Pasteur, in Paris, by Prof. Christine Petit's team (Institut de l'Audition, Institut Pasteur, IHU reConnect). The trial will assess the safety and efficacy of a new gene therapy drug in profoundly deaf children aged between 6 and 31 months.
February 2025