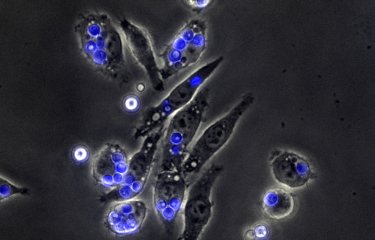
The fight against infectious bacteria requires the understanding at the molecular level of the defense strategies, often very elaborate, that they deploy to counteract and defuse the host's defenses. Since Metchnikoff's precursor work, special attention has been paid to the specific mechanisms by which bacteria can combat the acidity of phagosomes; vesicles inside which they are enclosed to be "digested" by the immune cells.
In the case of Mycobacterium tuberculosis (the agent responsible for tuberculosis), this defense was attributed to a virulence factor secreted by the bacteria, which could counteract the action of proton pumps, the purpose of which is precisely to create an acidic internal environment within the phagosomes. Research led by Priscille Brodin (Institut Pasteur in Lille and Korea) in collaboration with Edouard Yeramian and the team of Roland Brosch at the Institut Pasteur in Paris, Akihiko Yoshimura in Tokyo, and the P3M Platform of the LabEx ParaFrap reveal a new survival mechanism of the bacteria. This work, published in Cell Reports, shows that M. tuberculosis can divert a cellular route from the host to induce the expression of a protein (CISH), which attaches to the proton pump and leads to its degradation.
Beyond the potential therapeutic possibilities, this discovery opens perspectives in different directions. A functional synergy can be envisaged between the new and the previous models. Moreover, the defense mechanisms identified for the tuberculosis bacillus, or the declinations of these mechanisms, could intervene in other infectious pathologies, caused by bacteria or even parasites. It is woth noting that correlations have been observed in the literature between increased susceptibilities to different infectious diseases and genetic polymorphisms of CISH. Further studies should determine how these correlations can relate to the new model.
Source:
Mycobacterium tuberculosis controls phagosomal acidification by targeting CISH-mediated signalling
Christophe J. Queval (1,2), Ok-Ryul Song (1,3), Jean-Philippe Carralot (3), Jean-Michel Saliou (1,4), Antonino Bongiovanni (1), Gaspard Deloison (1), Nathalie Deboosère (1), Samuel Jouny (1), Raffaella Iantomasi (1), Vincent Delorme (1,3), Anne-Sophie Debrie (1), Park Sei-Jin (3), Joana Costa Gouveia (1), Stanislas Tomavo (1,4), Roland Brosch (2), Akihiko Yoshimura (5), Edouard Yeramian (6) and Priscille Brodin (1,3)
1 Univ. Lille, CNRS, Inserm, CHU Lille, Institut Pasteur de Lille, U1019 – UMR 8204 - CIIL - Center for Infection and Immunity of Lille, F-59000 Lille, France
2 Institut Pasteur, Unit for Integrated Mycobacterial Pathogenomics, F-75015 Paris, France
3 Institut Pasteur Korea, 16 Daewangpangyo-ro 712 beon-gil, Bundang-gu, Seongnam-si, Gyeonggi-do, 463-400, South Korea
4 Plateforme de Protéomique et Peptides Modifiés (P3M), CNRS, Institut Pasteur de Lille, Univ. Lille, F-59000 Lille, France
5 Department of Microbiology and Immunology, Keio University School of Medicine, 35 Shinanomachi, Shinjyuku-ku, Tokyo 160-8582, Japan.
6 Unité de Microbiologie Structurale, CNRS UMR3528 Institut Pasteur, 75015 Paris, France
DOI: 10.1016/j.celrep.2017.08.101