Vector-borne diseases: Mosquitoes, ticks, biting flies... how far will they go?

"We'll have to learn to live with the tiger mosquito" - Anna-Bella Failloux, entomologist at the Institut Pasteur (in french)
Copyright: Datagif & Olivier Roux - Institut Pasteur
Summary
Report - Mosquitoes, ticks, biting flies... how far will they go?
- A global issue
- Changing vector and human systems
- Climate, a key factor
- Vector-borne diseases soon in France?
Malaria - a disease of the past in Europe?
Leishmaniasis - a neglected disease that deserves more attention
Dengue - mapping towns and cities to combat this disease
Mobilization - The Institut Pasteur is mobilized during the 2024 Games
Ticks - in towns and cities: new areas that need to be monitored
Interview - Anna-Bella Failloux
Vector-Borne Diseases - A dedicated Research Center
Key figures - the CMTV
Profile - Marine Viglietta
For the past few years, warnings about the dangers of biting insects in spring have raised alarm bells in increasingly northerly regions. Over and above the irritation they cause, hematophagous insects and ticks represent a considerable health risk: in certain conditions, they are capable of hosting and transmitting viruses, parasites and bacteria, becoming disease "vectors."
Some "vector-borne" diseases are already a familiar problem in Europe, like Lyme disease, caused by ticks. Yet most such diseases – malaria, dengue, Zika, Crimean-Congo hemorrhagic fever and yellow fever, for example – may seem like a distant threat. But they are closer than we might care to think: insect vectors are taking advantage of human mobility, rising temperatures and habitat change to widen their range, automatically increasing the risk associated with vector-borne diseases.
As summer and the 2024 Games approach, particular attention is being paid to disease vectors such as mosquitoes, ticks and biting flies.
a disease of the past in Europe?
The most deadly vector-borne disease was present in several European regions until it was eradicated in the 1970s. "We have shown that some Anopheles mosquitoes on the continent were still capable of transmitting one of the parasites responsible for malaria," emphasizes Catherine Bourgouin, a group leader in the Biology of Host-Parasite Interactions Unit. "This indicates that the eradication of malaria in Europe cannot be attributed solely to a reduction in the number of vector mosquitoes; it also reflects improved socio-economic conditions and the existence of effective drugs. For example, before the rural exodus, Anopheles mosquitoes were able to over-winter in stables below bedrooms." So rather than a disease of the past, malaria should be considered as a rural disease. In Europe today, most cases of malaria are imported, and while indigenous cases are not impossible, like in Corsica in 2006, the conditions required for an outbreak are not yet fully in place. "Outside the summer season, Anopheles mosquitoes do not survive for long, which means that the parasite is unable to develop in their salivary glands," explains the scientist. "We have also shown that mosquitoes born to older parents are more susceptible to infection. This is an interesting avenue for research and raises hopes that we may be able to develop more effective means of preventing transmission of the parasite." In other world regions, vector control in rural areas, especially with the use of insecticide-treated bed nets, has led to a significant drop in malaria cases over the past 15 years. "But surveillance remains crucial in endemic countries: mosquitoes are adjusting their behavior to these strategies, adapting to polluted environments and extending their range to suburban areas, boosted by the rise in temperatures."
 Mosquitoes reared at the Institut Pasteur's Center for the Production and Infection of Anopheles. Each continent has its own species of Anopheles mosquitoes, many of which can be malaria vectors.
Mosquitoes reared at the Institut Pasteur's Center for the Production and Infection of Anopheles. Each continent has its own species of Anopheles mosquitoes, many of which can be malaria vectors.
A global issue
Although vector-borne diseases have long been seen as tropical diseases that are endemic among the world's poorest populations, the situation is changing rapidly: 80% of the global population is now at risk of one or more such diseases. According to the World Health Organization (WHO), they kill more than a million people every year, primarily children, and they often result in debilitating chronic conditions. The most deadly of these diseases is malaria, transmitted by Anopheles mosquitoes: in 2022, 249 million cases were reported worldwide, leading to 608,000 deaths, mostly on the African continent. Dengue is the most widespread vector-borne disease. The dengue virus is transmitted by Aedes mosquitoes, and since 2019 dengue has been present in every world region, putting half the world's population at risk. Outbreaks associated with other pathogens have been on the rise for the past decade, burdening health systems and spreading to new regions. New threats have also emerged, such as the Zika virus, which in 2016 was identified as being a cause of congenital malformations after major outbreaks in South America. Although a number of large-scale programs have been introduced to tackle vector-borne diseases, some are still considered by WHO as neglected diseases, including leishmaniasis and sleeping sickness.
a neglected disease that deserves more attention
Leishmaniasis, caused by parasites of the genus Leishmania, can lead to a wide variety of symptoms, from stigmatizing skin lesions to visceral damage that is fatal if left untreated. It is endemic in 90 countries, claims nearly 57,000 lives every year, and is considered as a neglected disease by WHO. "In the south of France, canine leishmaniasis, caused by Leishmania infantum, is particularly common," explains Gerald Spaeth, Head of the Molecular Parasitology and Signaling Unit. "But another very worrying strain of Leishmania, much more virulent than L. infantum, is emerging in Italy." The research by Gerald and his team has shown that this novel parasite is a hybrid between L. infantum and L. donovani, a Leishmania strain responsible for deadly outbreaks in India and West Africa. Similar hybrids have been described elsewhere in the Mediterranean. "Leishmania is a bit like a Frankenstein's monster," explains the scientist. "It is capable of sharing characteristics between different strains or adapting to very different environments and multiple species of sandflies, the insect vectors that transmit Leishmania to humans." These insects are benefiting from climate change and are spreading northwards at a growing rate, significantly increasing the risk associated with leishmaniasis in Europe. "Any neglected disease has the potential to become an emerging disease," explains Gerald Spaeth. "The unique, complex biology of Leishmania can create unpredictable epidemiological scenarios. We are exploring new therapeutic avenues to hinder the adaptability of these parasites by directly targeting their interaction with humans."

Changing vector and human systems
The mere presence of ticks or insect vectors is not the only condition required for the spread of vector-borne diseases; they need an effective "vector system" involving interactions between a pathogen, a vector and potential human or animal hosts. Understanding this system and the factors that influence it is crucial in combating vector-borne diseases. There are many reasons why these diseases are progressing, but major social and environmental changes are clearly playing a part. The disappearance of malaria from Europe, driven by the rural exodus, has much to teach us. Conversely, the urbanization of human societies can also have a favorable impact on some vector species. Dense urban centers provide them with new habitats and new means of transportation as they follow human mobility patterns, creating new epidemiological situations. Globalization and international travel are also helping to spread pathogens and vectors to all regions of the world. But vectors can only become established in a region if the climate conditions are favorable.
Mapping towns and cities to combat this disease
Dengue, once a tropical disease, has progressed dramatically over the past 20 years. According to WHO, there are some 100 to 400 million infections every year, and since 2019 they have occurred in every world region. "As well as climate, one of the factors driving this expansion is the development of greener urban centers that are more densely populated and more centralized, creating fertile ground for the mosquitoes that transmit dengue," explains Richard Paul, a scientist in the Ecology and Emergence of Arthropod-Borne Pathogens Unit. "Given the urbanization of mosquitoes, large-scale disinsection in areas around detected cases is no longer an effective strategy." So Richard Paul has been working with geographers, climatologists and epidemiologists to develop a novel approach that involves mapping towns and cities for more targeted vector control. "Based on data from the road network, social media, mosquito traps and samples taken, we can determine which urban areas are the busiest, the most conducive to mosquitoes, and the most likely to see dengue cases – three factors that are not necessarily always aligned, especially because of growing mobility within towns and cities." The method has been successfully applied in Delhi, India, paving the way for the adoption of targeted health policies, and it is currently being implemented in the Thai capital Bangkok. "Our research has led to the intelligent use of insecticides and also the development of early warning systems based on social and environmental data. Some European cities are already experimenting with these tools, which can be used to estimate the risk of an outbreak and take action at local level."

Climate, a key factor
Of all infectious diseases, vector-borne diseases are influenced most strongly by the rise in global temperatures. The impact of climate change may seem straightforward: extended warm seasons enable the majority of insects and ticks to reproduce more successfully and survive for longer. But while this is undoubtedly true for most species, the environmental changes caused by warming are more complex. For example, temperatures can modify a vector's ability to transmit a pathogen, and this interaction can change in response to the environment. Human activities and lifestyles are also affected by rising temperatures. "Blue and green" public policies are leading to the implementation of urban greening plans in towns and cities, and urban expansion is blurring the boundaries between built-up and rural areas, but local populations are not necessarily aware of the problem of ticks and insect vectors. These developments benefit both vectors and animal species that are reservoirs for vector-borne diseases. In France, this is the case for some rodents and birds that can host the bacteria responsible for Lyme disease and transmit them via ticks (Ixodes ricinus) to walkers. This cycle seems to have been amplified in recent years, with the number of annual estimated cases nearly doubling over the past decade.
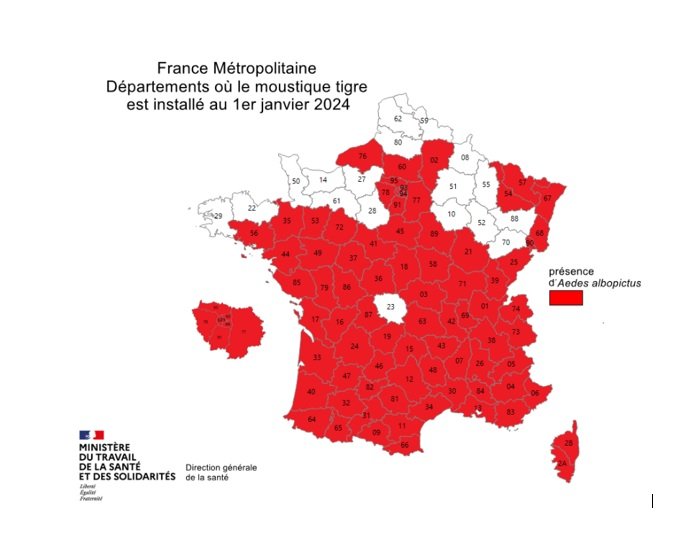
Vector-borne diseases soon in France?
In Europe, diseases like dengue, chikungunya and malaria are often thought of as "travel-related diseases," with cases imported by travelers or caused by infected mosquitoes accidentally introduced on flights from endemic regions. There are just a few hundred such cases each year in France, but local conditions are changing. Vectors are spreading across Europe, and the tiger mosquito, capable of transmitting viruses like Zika, dengue and chikungunya, has now colonized virtually all of mainland France (see map), increasing the probability that a local chain of transmission or even an outbreak may be kick-started by an imported case. While nine episodes of indigenous dengue transmission, in other words cases caused by the local mosquito population, were observed in France in the period from 2010 to 2018, the same number of cases were reported for a single year in 2022. The situation is such that in July 2022 the French Committee for Monitoring and Anticipating Health Risks (COVARS) emphasized that "the emergence and outbreaks of vector-borne diseases in France are inevitable." This observation may seem alarming, but it should be seen in the light of France's specific environmental and health context. Although pathogens have been introduced on multiple occasions, none has become established in the country, and vector activity remains highly seasonal. In short, the conditions are not yet in place to support the maintenance of a year-round vector-based system, but sporadic outbreaks are inevitable. So health authorities are on their guard: in France, a plan to prevent the spread of chikungunya and dengue has been in force since 2006. Preparedness is crucial, and effective means of vector control need to be developed. The considerable diversity and complexity of transmission cycles for vector-borne diseases complicates the task of developing vaccines and therapies. Vector control is currently the most effective weapon, and we can all play our part by using mosquito nets, wearing long trousers and paying particular attention after going for a walk in the woods!
The Institut Pasteur is mobilized during the 2024 Games
Since the beginning of 2024, a high number of imported cases of dengue fever have been recorded in France. Many tourists are flocking to France during the summer games, potentially bringing with them so-called "vector-borne" diseases. Imported cases of dengue fever could lead to set up a chain of transmission in mainland France. As a result, insect vectors such as tiger mosquitoes and ticks need to be monitored. For this, the Institut Pasteur's Cellule d'intervention biologique d'urgence (CIBU) is mobilized during the summer games. It is ready to intervene if suspected cases of fever or other symptoms are reported. Its role is to identify the pathogen causing the symptoms observed in order to prevent the outbreak of an epidemic.
in towns and cities: new areas that need to be monitored
In 2009, the estimated number of cases of Lyme disease in France was around 26,000. Today, nearly 50,000 people are affected each year by this debilitating disease, which can cause severe joint pain and neurological complications. "The geographical distribution of several tick species is changing, and in Europe ticks can now be found in new environments, including at higher altitudes because of rising temperatures and in urban and peri-urban green areas," explains Eva Krupa, a scientist in the Ecology and Emergence of Arthropod-Borne Pathogens Unit. "This increasingly brings tick populations into contact with people who are not aware of the risks they represent, especially on routes through green areas that are popular not only with walkers but also with animals, which themselves can be reservoirs for pathogens." With her team, Eva is trying to improve our understanding of the risks associated with these environments by collecting ticks from the various populations in the Greater Paris region and gathering data about their bites and the diseases they can cause. The risk map developed on the basis of this research will enable health authorities to introduce local preventive measures – but the scientist wants to go further. "As part of a European program, we are also developing a "lab in a suitcase" to detect pathogens directly in the field and respond rapidly in the event of an outbreak." The project has taken on a new resonance since the detection of novel tick-borne pathogens in France, like the pathogen responsible for Crimean-Congo hemorrhagic fever in October 2023.


"Ticks are fearsome for both humans and animals" - Sarah Bonnet, Head of the Ecology and Emergence of Arthropod-borne Pathogens unit (in french)
Copyright: Jeanne Fenouil/ Institut Pasteur

"We knew that climate change would exacerbate vector-borne diseases, but a situation that is so favorable to disease outbreaks is a new development."
Are mosquitoes our enemies?
No, but the pathogens they can carry are. Just 15% of the more than 3,500 recorded species of mosquitoes bite humans, and even fewer are responsible for disease outbreaks. But pathogens always arrive once the insect vector is well established. The scenario is the same, regardless of whether it takes a few years or a decade. So we cannot take the current situation lightly: the establishment of the tiger mosquito, Aedes albopictus, and human mobility are conducive to emerging disease outbreaks. Every pathogen obviously has its own characteristics: some are transmitted by Culex mosquitoes that bite birds (the West Nile and Usutu viruses), but the most dangerous have humans as their main reservoir. Of these pathogens, only viruses have pandemic potential because of both their ability to adapt and multiply rapidly and the expanding geographical range of the insects that transmit them.
Why are they so successful?
Like all insects, mosquitoes are cold-blooded creatures whose internal temperature depends on the external temperature. So there is a direct correlation between climate change and the activity and density of mosquitoes: they are developing more quickly, and the virus incubation period is also becoming shorter. The higher the temperature, the greater the risk associated with vector-borne diseases. Outbreaks occur most often in towns and cities, and some species, especially the genus Aedes, have adapted particularly well, becoming "urbanized" and taking advantage of breeding grounds provided by humans such as gutters, flower pots and water storage containers.
Is that why the tiger mosquito can now be found in France?
Precisely! The tiger mosquito is the ultimate invasive species: its eggs have a thick waterproof shell that can withstand both drought and temperatures of 0°C. This has allowed the mosquito to follow human movements, arriving in Europe from the United States, probably in used tires transported by container ship. In France, the tiger mosquito arrived in Marseille in 2010 and made its way up the Rhône Valley via vehicles on the highway. Its geographical range already covers around 20 European countries, and it will continue to spread.
What risk does it represent?
With travelers returning from tropical countries and importing viruses that Aedes albopictus can carry, especially dengue, chikungunya and Zika, the ongoing risk of indigenous cases is clear. The scientific community knew that climate change would exacerbate vector-borne diseases, but a situation that is so favorable to disease outbreaks is a new development. It became a reality in 2007 with the detection of the first indigenous cases of chikungunya in Italy: in less than a day, one person had generated 250 secondary cases! It gained momentum very quickly, and the outbreak only stopped when the transmission season came to an end. That is the advantage we have in Europe – we have a winter season, unlike overseas territories.
How can we deal with this situation?
France has a well developed vector control policy. A plan to prevent the spread of chikungunya and dengue was introduced in 2006 and runs from May 1 to November 30 each year. If an indigenous case is detected, all areas at risk of transmission are disinsected. But we are faced with the problem of resistance to insecticides, especially deltamethrin, the only insecticide authorized in Europe against adult mosquitoes. Vector control is limited but crucial, as we have few therapeutic and vaccine options against the viruses themselves. The best way to avoid falling ill is to protect ourselves against mosquitoes. They are no longer just a source of irritation; they represent a real health risk.
A dedicated Research Center
Vector-borne diseases are infections transmitted to humans by vectors, usually animals such as mosquitoes, ticks, or fleas, which carry and transmit pathogens. With climate change, the range of these animals is expanding, increasing the risk associated with the diseases they transmit. The aim of the Institut Pasteur's future Research Center for Vector-Borne Diseases (CMTV), which will soon be built on the Paris campus, is to study the different dimensions of these vector-borne diseases by bringing together specialists in all aspects of the host–vector–pathogen triad under the same roof. "Around 15 teams will be hosted in this building, the only center of its kind in Europe," explains Philippe Bastin, scientific coordinator of the project and Head of the Trypanosome Cell Biology Unit. "The Institut Pasteur has a long history* of expertise in vector-borne diseases and a global reputation in this field. Our teams are already actively working in response to this new threat, and the research center will give them fresh impetus to expand the boundaries of knowledge, for the benefit of public health. The aim is to be able to work as closely as possible to the natural infection cycle of human pathogens," he continues. "We can observe this cycle at different scales, from the perspective of genetics, the immune system or the environment." The center will have state-of-the-art imaging tools so that scientists can monitor viruses, bacteria and parasites in the body of the vector or host at every level, from organ to cell. Insectariums with flight tunnels will be used to study how the behavior of vectors is influenced by their environment and by infection. Finally, this infrastructure will enable us to continue our work of discovering emerging pathogens in collaboration with our international partners throughout the world, particularly within the Pasteur Network. "We want to create a virtuous cycle linking basic research and field-based medical research."

* The malaria parasite and the plague and typhus bacilli were discovered by Institut Pasteur scientists in the early 20th century. Research on insects is a long-standing tradition at the Institut Pasteur: the course on medical entomology has provided training for experts worldwide for more than 35 years.
Marine Viglietta, from the Caribbean to Asia

Marine Viglietta
Research is just a roundabout way of helping others.
Marine Viglietta, 27, a medical entomologist and virologist, completed her PhD in the Arboviruses and Insect Vectors laboratory at the Institut Pasteur. She talks enthusiastically about her research on Aedes aegypti mosquitoes and the viruses they can spread.
When we meet Marine in the laboratory where she works, we find her with pipette in hand, waving at us to wait while she finishes off a procedure. It's a race against the clock for the young scientist – she only has a few weeks left to finish her experiments before she begins writing up her thesis, the culmination of four years of work. She will defend her thesis in September. "I need a holiday!" exclaims Marine, grinning, as she joins us. The smile rarely leaves her face, especially when she is talking about her research.
A few years ago, she began studying medicine, attracted by the idea of being able to "help people" with a job that she finds "admirable." But she soon realized that medicine was not the right path for her – research was where her interests lay. She began by studying epidemiology, then virology at Université de Versailles Saint-Quentin-en-Yvelines. "I wanted to work on epidemic diseases, especially tropical diseases, as I spent a lot of time in the Caribbean when I was little, in Martinique and Guadeloupe, where there were mainly dengue outbreaks. That made me aware of vector-borne diseases transmitted by mosquitoes."
During a Master's internship in the Entomology Unit at the Institut Pasteur de la Guadeloupe, she had a lightbulb moment. "It was the first time that I had handled mosquitoes, and I learned how to rear them. That made me really want to work with these insects."
 Head of an Aedes mosquito
Head of an Aedes mosquito
For her PhD, Marine answered a call for applications from Anna-Bella Failloux's laboratory at the Institut Pasteur in Paris, which she had "had her eye on for a while." The call asked a simple question: why is there no yellow fever in Asia? "There is no yellow fever despite the fact that the mosquito vector for the disease is present in Asia!" explains Marine. In this research unit that studies mosquito-borne viruses – "arboviruses" –, Marine discovered what it was like to work in a high-security BSL3 laboratory, which required prior training. "I was able to handle arboviruses and work on my own project."
In 2022, she was sent for a few weeks to the Institut Pasteur du Cambodge to learn how to collect mosquitoes in the field. "We caught mosquitoes with traps, in Phnom Penh and in the rural village of Koh Thom. It was very interesting to see what things were really like on the ground – how people live alongside animals and insects. It was an amazing, memorable experience, also from a scientific perspective. But if I had to choose between the lab and the field, I would choose the lab every time. Working in the field is tough and unpredictable – sometimes you don't catch anything."

In the lab, Marine says that she loves seeing her experiments through to the end. "It often takes at least three months before we get any results: we need to rear and infect the mosquitoes, process the samples and then analyze them – it's a lengthy procedure, and I find it fascinating."
On yellow fever in Asia, Marine was able to show that if Aedes aegypti mosquitoes are infected with Japanese encephalitis virus, which is common in this region, it makes them unable to transmit yellow fever virus. The dengue virus also seems to have the same blocking effect. With these first results, Marine has begun to shed light on what was previously "one of the major mysteries of arbovirology."

Once she has defended her thesis and has her PhD, she will set off for a postdoctoral fellowship abroad, but she hasn't yet explored where she might go.
Does she still want to help others? "Research is just a roundabout way of doing so," says the young scientist. "Understanding how things work will help someone some day to find a vaccine or a way of blocking a virus in mosquitoes, and that would prevent hundreds of thousands of people from being infected. It's that prospect that drives me."
Fondation Crédit Mutuel Alliance Fédérale and the Institut Pasteur combat vector-borne diseases
Among the many research projects being carried out at the Institut Pasteur, one is studying tens of thousands of mosquitoes collected in every region of France, measuring their density and their ability to transmit around 13 different viruses in order to anticipate their emergence. Ultimately, the data collected will be used to draw up epidemic risk maps to guide the decisions taken by the health authorities.
This work is supported by the Fondation Crédit Mutuel Alliance Fédérale. We would like to thank it for its generous support and its commitment to our work.
Learn more: "Climate and health: anticipating tomorrow's vector-borne diseases in France"


