COVID-19: which monoclonal antibodies should be used for vulnerable individuals?

Vaccination does not offer sufficient protection against severe forms of COVID-19 for people with a weakened immune system. For these individuals, monoclonal antibodies have long been the preferred therapeutic solution. But depending on the SARS-CoV-2 variant, the effectiveness of these treatments has fluctuated. Scientists have had to develop novel antibodies that are more effective while also regularly evaluating those already in use. An Institut Pasteur team has been helping healthcare providers to determine the most suitable treatment strategies for their patients.
Summary
Foreword - Background
Definition - What are monoclonal antibodies?
Chronology - Characterizing monoclonal antibodies that are effective against SARS-CoV-2 variants
Omicron - Omicron variant: "almost a new pandemic"
Studies - The evolution of scientific studies at the Institut Pasteur
Collaboration - Essential collaboration between Institut Pasteur scientists and clinicians
Today - Monoclonal antibodies, still a relevant therapeutic solution
On October 1, 2020, US President Donald Trump was revealed to have tested positive for SARS-CoV-2. At the time there was no vaccine available and no antiviral treatments had shown compelling efficacy. The following day, the White House physician announced that Donald Trump had received an experimental cocktail of monoclonal antibodies, casirivimab/imdevimab. With this announcement, the general public discovered that the "next generation" messenger RNA vaccines that were about to be brought to market may not be the only means of protection against COVID-19.
A few weeks later, the first SARS-CoV-2 vaccines were granted marketing authorization in several countries, including in the European Union. They were reliable, offered strong protection and could be rapidly produced at scale, and they would go on to save millions of lives – nearly 20 million in 2021 alone, according to WHO. But for many people, vaccination proved to be an imperfect solution.
This was particularly the case for immunocompromised people (individuals with AIDS or cancer, transplant recipients or those with chronic illnesses, etc.), since their immune system is not sufficiently stimulated by vaccination. To avoid severe forms of COVID-19, they have to abide by health and hygiene measures like wearing masks and airing closed spaces even more scrupulously – a challenge at a time when the majority of people are starting to forget about them. Another possibility for immunocompromised individuals is treatment with an alternative therapeutic solution, the same one given to the former US President: the administration of monoclonal antibodies.
What are monoclonal antibodies?
Around thirty monoclonal antibodies used to treat a range of diseases (cancer, autoimmune diseases and infectious diseases) are currently authorized in France. Monoclonal antibodies are obtained in the laboratory from a single lineage – a single clone, hence the adjective in their name – of immune cells, specifically B cells. Once the gene encoding the antibody of interest has been selected, it is inserted into "cell factories" to produce the monoclonal antibody at scale. The effectiveness of treatments based on monoclonal antibodies depends on their ability to bind to a specific target. Monoclonal antibodies used to treat infectious diseases recognize a specific part of the microbe – the antigen – and bind to it. This means that the microbes are unable to infect the body's cells, or alternatively, if the antigen is expressed at the surface of an infected cell, the immune system is stimulated to specifically destroy that cell. The names of therapeutic antibodies all end in "mab," which stands for "monoclonal antibodies."
For COVID-19, monoclonal antibodies were designed to prevent SARS-CoV-2 entering the cell, by targeting the virus' spike protein. The spike protein, which gives SARS-CoV-2 its spiky appearance (the "corona" of coronaviruses), can be seen as the key that enables the virus to open the door to the cells in our body. By binding to the spike protein and stopping the cell from being infected, monoclonal antibodies neutralize the biological effects of the virus.
Characterizing monoclonal antibodies that are effective against SARS-CoV-2 variants
Several monoclonal antibodies, developed within a few months and authorized in late 2020 or early 2021 (depending on the country), initially demonstrated high protective efficacy against SARS-CoV-2. In France, two dual therapies for use in people at high risk of developing a severe form of COVID-19 were authorized in the first quarter of 2021. The casirivimab/imdevimab combination mentioned above was authorized as a preventive treatment from August 2021. Unfortunately, several SARS-CoV-2 variants (Alpha, Beta and Gamma) emerged in late 2020 and then spread across the globe. The Delta variant began circulating worldwide from spring 2021. What are the consequences of these mutated viruses for our immune response? Are the vaccines still effective? Do monoclonal antibodies still represent a relevant therapeutic solution? These are some of the main questions that scientists and clinicians have been exploring since the emergence of the variants, and the answers are crucial, especially when it comes to treating immunocompromised people.
"When the Delta variant emerged, we started to test the antibodies on the market and those that were in the advanced stages of preclinical trials," explains Timothée Bruel, a scientist in the Virus and Immunity Unit. The scientists demonstrated in summer 2021 that one of the antibodies tested, bamlanivimab, which had been effective against the original SARS-CoV-2 strain, was no longer effective against the Delta variant. Nevertheless, at the time, "there were still monoclonal antibodies that were working perfectly well against Delta, with results obtained on the original strains that could be extrapolated to the new variant," so there was relatively little concern at that stage. But the emergence of the Omicron variant in autumn 2021 changed everything.
Omicron variant: "almost a new pandemic"
Omicron is more transmissible, and it soon replaced the previous variants. In genomic terms, it also differed to a greater extent from the original virus. "While previous variants had fewer than ten mutations on the spike protein compared with the original strain, Omicron had more than thirty. It was almost as if a new pandemic had begun. With the emergence of this variant, we realized that vaccination offered less protection and that a third dose was needed to immunize the population. What we were less aware of was that Omicron had a significant ability to evade monoclonal antibodies," says Timothée Bruel. This was demonstrated by several scientists in an article published in December 2021. In their research, the scientists showed that two thirds of the monoclonal antibodies used in clinical practice or currently under development lost all their antiviral activity against Omicron. The situation also became increasingly complex as several subvariants (BA.1, BA.2, etc.) emerged over the following weeks. So the scientists tried to determine whether the monoclonal antibodies were effective against the new strains that were co-circulating. In a study published in March 2022, they provided a number of answers. The casirivimab/imdevimab combination, which until then had offered a similar level of protection against severe forms of COVID-19 as vaccination, was no longer active against Omicron. The neutralizing activity of another antibody cocktail (tixagevimab/cilgavimab) was significantly reduced against BA.1 compared with the Delta variant, but its neutralizing activity was not reduced nearly as much against BA.2. This result, which showed that a monoclonal antibody could lose its neutralizing capability for a given variant before recovering it for a subsequent variant, emphasizes the importance of continuing with efficacy testing on all available antibodies.
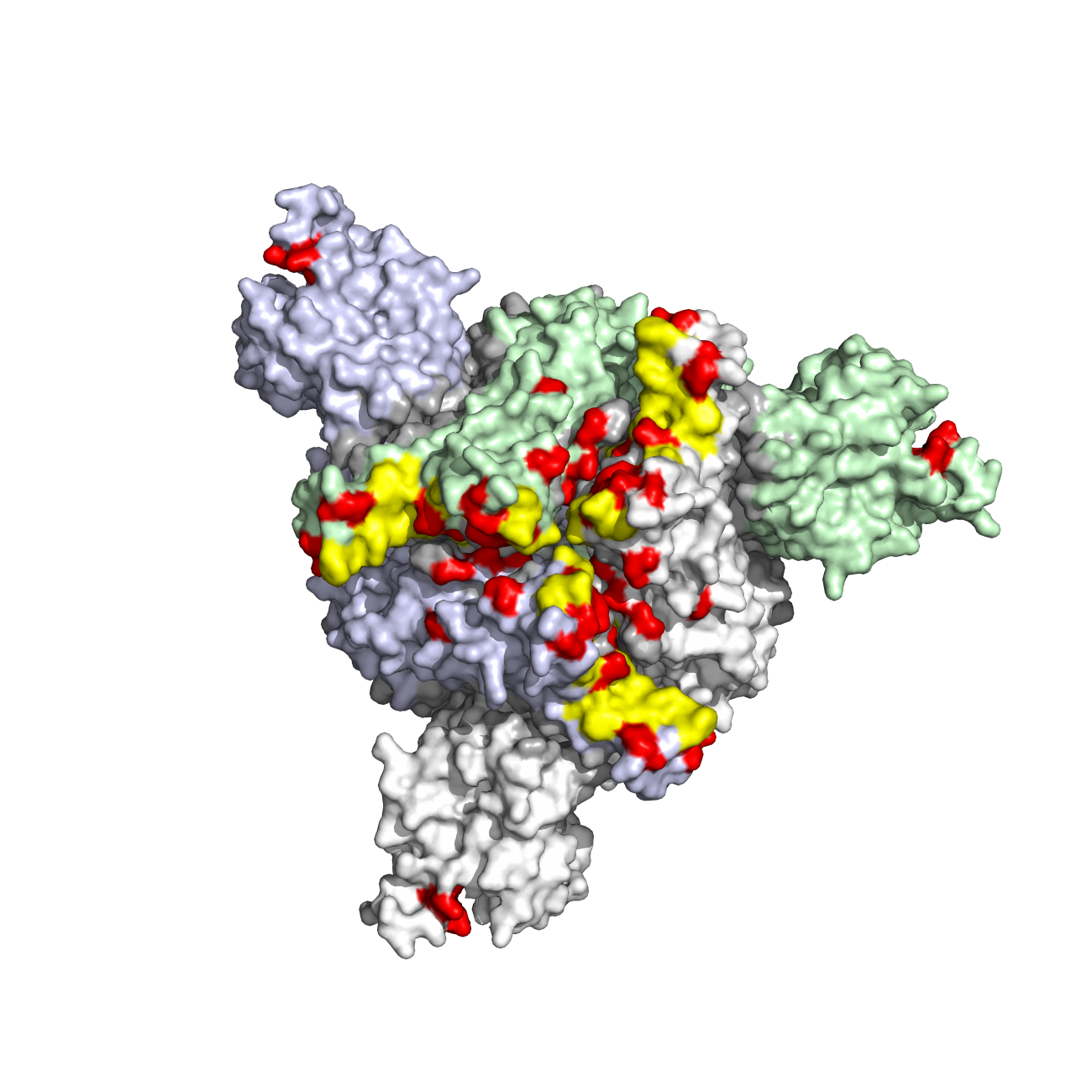
Caption: 3D visualization of mutations in the spike protein of the Omicron variant, seen from above. Mutations are indicated in red. Credit: Institut Pasteur/Olivier Schwartz & Félix Rey
Over the following months, the Institut Pasteur's scientists continued this painstaking task and even took it a step further. As well as in vitro studies, the scientists were determined to continue with in vivo studies using sera from people who had received monoclonal antibodies.
They also added new criteria to their research. The protective efficacy of monoclonal antibodies depends primarily, but not solely, on their neutralizing capability. Some monoclonal antibodies can also induce a key immune defense mechanism known as antibody-dependent cellular cytotoxicity (ADCC), which kills cells infected with SARS-CoV-2. In the case of COVID-19, natural killer (NK) cells in the immune system recognize the antibodies that bind to antigens on the surface of infected cells and then specifically lyse those cells. These antibodies are described as "polyfunctional." This characteristic is all the more interesting since the monoclonal antibodies with the highest neutralizing capability are not those that induce ADCC the most, as shown in a study published in December 2022. In short, to obtain protective antibody cocktails, it may be a good idea to include polyfunctional antibodies which combine a high neutralizing capability with a strong ability to induce immune defense mechanisms.
Essential collaboration between Institut Pasteur scientists and clinicians
Cooperation with clinician partners is crucial for the wide-ranging COVID-19 research performed by the Virus and Immunity team. These partners include Orléans Regional Hospital, Georges Pompidou European Hospital, Pitié-Salpêtrière University Hospital and Toulouse University Hospital. In some cases the collaborative work has been ongoing for many years. "I have been working with Olivier Schwartz's unit for more than ten years. Our first joint research was on HIV, especially the mechanisms by which the virus is transmitted from one cell to the next. Our working relationship became much closer during the COVID period because it was clear that our expertise was complementary and that our respective research could benefit from working together," explains Anne-Geneviève Marcelin, a virology professor in the Sorbonne University Faculty of Health and Head of the Virology Department at Pitié-Salpêtrière Hospital. "We bring our vision, with the questions that arise in a very practical way for treating patients, and we also offer the possibility of working on samples collected from patients as part of our research. So this collaboration is a way of linking basic research with applications for patients," she continues.
What did that mean in practice for COVID-19? "The data generated by the Virus and Immunity Unit and their colleagues showed that sotrovimab, unlike other monoclonal antibodies, maintained ex vivo antiviral activity on the Omicron subvariants of interest BQ.1.1 and XBB.1.5, especially because of the preserved immunological functions of sotrovimab," explains Guillaume Martin-Blondel, a professor at Toulouse University Hospital and the Toulouse Institute for Infectious and Inflammatory Diseases. "While the variants were circulating, these experimental data meant that we were able to identify sotrovimab as a curative treatment for non-severe forms of COVID-19 if other therapeutic alternatives could not be used." This is a clear illustration of the essential links between laboratory research and clinical research that help shed light on crucial public health questions.
Monoclonal antibodies, still a relevant therapeutic solution
What is the situation today? Given their loss of efficacy, there are currently no monoclonal antibodies that are approved for preventive use. For infected individuals who may develop severe forms, the recommended treatments are antiviral drugs like the nirmatrelvir/ritonavir combination or remdesivir. But these drugs have some drawbacks: they may interact with other medication, they can have significant side effects, and they are not easy to administer, so as a therapeutic option they are limited. Where do monoclonal antibodies stand as a curative treatment? While sotrovimab has been recognized as a possible alternative for the SARS-CoV-2 variants that were circulating in early 2023, it is ineffective against some of the current variants.

Cells that have fused and formed syncytia in green, spike production in red, cell nuclei in blue. Credit: Institut Pasteur
230,000 to 300,000 people in France alone are immunocompromised and at risk of developing severe forms of COVID-19.
So can we say that monoclonal antibodies no longer serve any purpose? No, since research is continuing to produce new, more effective monoclonal antibodies. In convalescent individuals, a team of scientists coordinated by Hugo Mouquet has identified powerful broadly neutralizing antibodies that are active for a wide range of SARS-CoV-2 variants. The corresponding monoclonal antibodies are currently under development by SpikImm, a start-up founded by Truffle Capital and the Institut Pasteur. SpikImm has already taken two of these antibodies through to Phase I completion and is now developing an optimized antibody that will be more effective against the current variants, which could soon be approved for clinical practice. Depending on the results, the antibodies developed could be fast-tracked for authorization to be used preventively against COVID-19 in immunocompromised individuals. Other monoclonal antibodies against new variants, developed by various pharmaceutical companies, are also undergoing clinical trials.
Finally, other potential strategies for the development of future monoclonal antibodies for COVID-19 are under exploration. Scientists are now aiming to prevent SARS-CoV-2 from entering the cell by obtaining neutralizing antibodies that target the spike protein. But this protein undergoes numerous mutations, considerably reducing the effectiveness of antibody recognition. Can other parts of the virus be targeted? "One might imagine that antibodies can be produced to target other viral proteins, and that these antibodies might have other functions than neutralization. But this requires a better understanding of the functions of monoclonal antibodies. Research in this area could help us expand our antibody toolkit so that we are more resilient when dealing with viral diversification," indicates Timothée Bruel.
So in this "reciprocal adaptation" between monoclonal antibodies and viral variants, scientists have not yet played their last card. This is a priority, since 230,000 to 300,000 people in France alone are immunocompromised and at risk of developing severe forms of COVID-19.
Sources:
Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization, Nature, July 8, 2021
Considerable escape of SARS-CoV-2 Omicron to antibody neutralization, Nature, December 23, 2021
Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies, Nature Medicine, March 23, 2022
Potent Human Broadly SARS-CoV-2 Neutralizing IgA and IgG Antibodies Effective Against Omicron BA.1 and BA.2, Journal of Experimental Medicine, June 15, 2022
Longitudinal analysis of serum neutralization of SARS-CoV-2 Omicron BA.2, BA.4, and BA.5 in patients receiving monoclonal antibodies, Cell Reports Medicine, December 20, 2022
Sotrovimab therapy elicits antiviral activities against Omicron BQ.1.1 and XBB.1.5 in sera of immunocompromised patients, Med, October 13, 2023