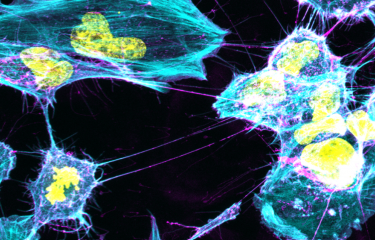
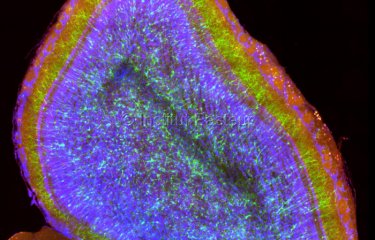
Over the last few decades, neurodegenerative diseases became one of the top 10 global causes of death. Researchers worldwide are making a strong effort to understand neurodegenerative diseases pathogenesis, which is essential to develop efficient treatments against these incurable diseases. However, our knowledge about the basic molecular mechanisms underlying the pathogenesis of neurodegenerative diseases is still lacking. A team of researchers found out the implication of lysosomes in the spread of Parkinson’s disease.
The accumulation of misfolded protein aggregates in affected brain regions is a common hallmark shared by several neurodegenerative diseases (NDs). Mounting evidence in cellular and in animal models highlights the capability of different misfolded proteins to be transmitted and to induce the aggregation of their endogenous counterparts, this process is called “seeding”. In Parkinson’s disease, the second most common ND, misfolded α-synuclein (α-syn) proteins accumulate in fibrillar aggregates within neurons. Those accumulations are named Lewy bodies.
α-syn fibrils spreads through TNTs inside lysosomes
In 2016, a team of researchers from the Institut Pasteur (Paris) and the French National Centre for Scientific Research (in French: CNRS, Centre national de la recherche scientifique) demonstrated that α- syn fibrils spread from donor to acceptor cells through tunneling nanotubes (TNTs). They also found out that these fibrils are transferred through TNTs inside lysosomes. “TNTs are actin-based membrane channels allowing the transfer of several cellular components including organelles between distant cells. Lysosomes are organelles normally deputed to the degradation and recycling of toxic/damaged cell material” describes Chiara Zurzolo, head of the Membrane Traffic and Pathogenesis Unit at the Institut Pasteur.
α-syn fibrils can modify lysosome shape and permeability to allow seeding and diffusion
Following this original discovery, researchers, now shed some light on how lysosomes participate in the spreading of α-syn aggregates through TNTs. “By using super-resolution and electron microscopy, we found that α-syn fibrils affect the morphology of lysosomes and impair their function in neuronal cells. We demonstrated for the first time that α-syn fibrils induce the peripheral redistribution of the lysosomes thus increasing the efficiency of α-syn fibrils’ transfer to neighbouring cells,” continues Chiara Zurzolo. They also showed that α-syn fibrils can permeabilize the lysosomal membrane, impairing the degradative function of lysosomes and allowing the seeding of soluble α-syn, which occurs mainly in those lysosomes. Thus, by impairing lysosomal function α-syn fibrils block their own degradation in lysosomes, that instead become a hub for the propagation of the pathology.
by impairing lysosomal function α-syn fibrils block their own degradation in lysosomes, that instead become a hub for the propagation of the pathology
This discovery contributes to the elucidation of the mechanism by which α-syn fibrils spread through TNTs, while also revealing the crucial role of lysosomes, working as a Trojan horse for both seeding and propagation of disease pathology. This information can be exploited to establish novel therapies to target these incurable diseases.
This study is part of the priority scientific area Brain connectivity and neurodegenerative diseases of the Institut Pasteur's strategic plan for 2019-2023.
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 897378
Source:
α-Synuclein fibrils subvert lysosome structure and function for the propagation of protein misfolding between cells through tunneling nanotubes, Plos Biology, July 20 2021
Aysegul Dilsizoglu Senol1§, Maura Samarani1§, Sylvie Syan1, Carlos M. Guardia2, Takashi Nonaka3, Nalan Liv4, Patricia Latour Lambert5, Masato Hasegawa3, Judith Klumperman4, Juan S. Bonifacino2, Chiara Zurzolo1*
1Unité de Trafic Membranaire et Pathogénèse, Département de Biologie Cellulaire et de l’Infection, Institut Pasteur, Paris, France.
2Neurosciences and Cellular and Structural Division, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
3Dementia Research Project, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan.
4Section Cell Biology, Center for Molecular Medicine, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands.
5Dynamique des Interactions Hôte-Pathogène, Département de Biologie Cellulaire et de l’Infection, Institut Pasteur, Paris, France.
§These authors contributed equally