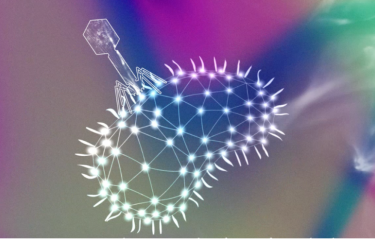
Membrane vesicles (also known as extracellular vesicles) are produced by all kinds of organisms. They are small lipid bags that come off a donor cell and get internalized by a recipient cell. Their role is to carry biological information and they are described as key intercellular communication players. However, their role in the microbial world is still very much unknown. Researchers from the Institut Pasteur and Arizona State University examined Membrane Vesicle movements in bacterial populations.
Though the words MVs (Membrane Vesicles) might seem foreign to many, they are currently utilized to package out the SPIKE mRNA in the long awaited Covid19 vaccine. Their nanoparticle size enables their dissemination through numerous tissue types, opening exciting new possibilities for their application in therapeutic and vaccine design.
Extensive investigation and outstanding discoveries in the field of MV transport and functions in Eukaryotic cells was awarded the Nobel Prize in Physiology and Medicine in 2013. In the microbial world, the importance of MVs lacks experimental evidence and the dynamical aspects remains unexplored.
A chance discovery leads to unprecedented work
“I was not looking for membrane vesicles when I first came to visualize and image them under the microscope,” explains Julia Bos, research engineer at the Bacterial Genome Plasticity Unit at the Institut Pasteur (Paris). “I have been imaging a whole palette of bacterial strains throughout my career path, with the goal of elucidating mechanisms underlying the emergence of antibiotic resistance… but my eyes had never caught membrane vesicles! I found them by chance, with the right amount of dye, the fortunate magnification and the fine microscopy setup.” She spotted membrane bags undergoing a random walk along the surfaces of filamentous E. coli bacteria challenged with low doses of antibiotics.
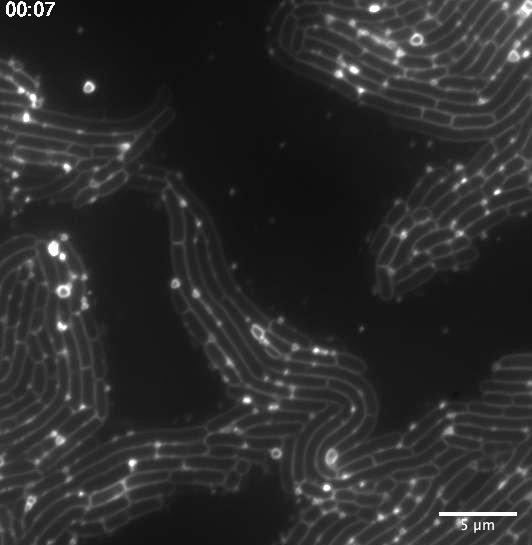
Copyright : Julia Bos/Institut Pasteur
Membrane vesicle enhanced motion might be a response to antibiotic stress
After this discovery, Julia Bos, with Didier Mazel in whose research group the work was done, and Luis Cisneros, faculty member at the Beyond Center at ASU in Tempe, USA, studied the mechanism. Using fluorescence microscopy, they provided a quantitative picture of the membrane vesicle dispersion and location within a bacterial population. They determined MV motion properties could possibly be a signature of antibiotic stress.
“There is a lot more to discover given the diversity of microbial species and heterogeneity in vesicle size and composition“ concludes Julia Bos. This work provides exciting insights in the field of membrane vesicles and their role in the microbial world.
Source
Real-time tracking of bacterial membrane vesicles reveals enhanced membrane traffic upon antibiotic exposure, Science advances, 20 January 2021
Julia Bos1*†, Luis H. Cisneros2*†, Didier Mazel1
1Unité Plasticité du Génome Bactérien, Institut Pasteur, UMR3525, CNRS, Paris 75015, France.
2The Biodesign Center for Biocomputing, Security and Society, and BEYOND Center for Fundamental Concepts in Science, Arizona State University, Tempe, AZ, USA.
†Corresponding author. Email: julia.bos@pasteur.fr (J.B.); lhcisner@asu.edu (L.H.C.)
* These authors contributed equally to this work.