Scientists from the Institut Pasteur and the CNRS have recently identified new strategies used by Helicobacter pylori bacteria to infect cells. By specifically targeting mitochondria these bacteria, despite being extracellular, can optimize infection in the host. These findings pave the way for new strategies to combat H. pylori infection, which is associated with most cases of gastric cancer and several other gastric disorders. The results of this research were published on November 21 in the journal Scientific Reports.
Helicobacter pylori is a bacterial pathogen that colonizes the stomach of approximately half of the world's population. Infection with H. pylori is acquired in childhood and lasts for decades. H. pylori is the main risk factor for gastric cancer and is linked to more than 80% of cases. Gastric cancer, the third most common cause of cancer-related death, is often associated with a poor prognosis because it tends to be diagnosed at an advanced stage. It is responsible for about 800,000 deaths each year worldwide.
H. pylori has several virulence factors that interact with specific targets in the cell and directly affect the severity of gastric disease. Vacuolating cytotoxin A (VacA) was previously the only main H. pylori factor known to act on mitochondria*, causing cellular membrane and organelle dysfunction and leading to cell death.
Scientists from the Institut Pasteur and the CNRS have discovered that H. pylori uses at least two additional strategies to target mitochondria. These strategies do not lead to cell death but maintain an environment that is conducive to bacterial proliferation.
Their results show that H. pylori affects both mitochondrial transport systems (used to transfer proteins into mitochondria) and the machinery for the replication and maintenance of the mitochondrial genome. The scientists also discovered that, contrary to what was previously believed, VacA is not the only H. pylori component capable of affecting mitochondria. This suggests that the bacteria may produce other mitochondria-interacting factors that have not been yet identified.
As Miria Ricchetti, joint last author of the paper and a scientist at the Institut Pasteur**, explains, "the damage to mitochondria caused by H. pylori bacteria is temporary and disappears once the infection has been eliminated. Despite remarkably high levels of stress, mitochondria, like cells, can remain functional and withstand infection for longer than previously thought. It is important for us to bear this in mind when looking for strategies to inhibit the bacterium's pathogenic potential."
Eliette Touati, joint last author of the paper and a scientist at the Institut Pasteur**, adds: "We have observed in a mouse model that this type of damage is associated with a worsening of gastric lesions. The damage may therefore affect the chronicity and severity of infection by H. pylori. Understanding these new interactions between pathogen and host cells (via mitochondria) is vital for the development of effective strategies to combat H. pylori infection. The aim is to reduce the persistence of the bacteria in the stomach and curb associated conditions, especially cancer."
* Mitochondria are cell "powerhouses" that produce the cell's energy (ATP). They are also involved in other essential cell functions.
** Miria Ricchetti leads the Stability of Nuclear and Mitochondrial DNA team in the Stem Cells and Development Unit.
Eliette Touati leads the Infection, Genotoxicity and Cancer team in the Helicobacter Pathogenesis Unit.
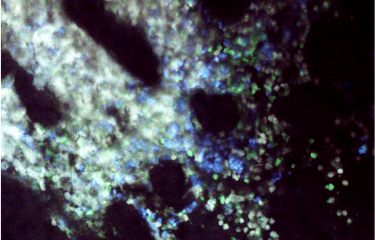
Image : Gastric tissue infected by H. pylori. Nuclei are in blue (Hoechst) and mitochondria in red (Mitotracker labelling).
Funding :
Odyssey Reinsurance Company, Association pour la Recherche sur le Cancer ARC.
Source
Helicobacter pylori targets mitochondrial import and components of mitochondrial DNA replication machinery through an alternative VacA-dependent and a VacA-independent mechanisms, Scientific Reports, November 21st, 2017
Laurent Chatre (1,2), Julien Fernandes (3,4), Valérie Michel (3,4), Laurence Fiette (5,6), Patrick Avé (5,6), Giuseppe Arena (1,2,7,8), Utkarsh Jain (9), Rainer Haas (9,10), Timothy C Wang (11), Miria Ricchetti (1,2) et Eliette Touati (3,4)
1 Cellules souches et développement, Institut Pasteur, 25-28 Rue du Dr Roux, Paris, France
2 CNRS UMR3738, groupe Stabilité de l’ADN nucléaire et mitochondrial, Paris, France
3 Unité Pathogenèse de Helicobacter, Institut Pasteur, 25-28 Rue du Dr Roux, Paris, France
4 CNRS ERL3526, groupe Infection, génotoxicité et cancer, Paris, France
5 Unité Histopathologie humaine et modèles animaux, Institut Pasteur, 25-28 Rue du Dr Roux, Paris, France
6 Université Paris Descartes, PRES Sorbonne Paris Cité, Paris, France
7 IRCM (Institut de recherche en cancérologie de Montpellier), Université de Montpellier, 34298, France
8 INSERM U1194, Montpellier, France
9 Max von Pettenkofer-Institut für Hygiene und Medizinische Mikrobiologie, Ludwig-Maximilians-Universität, Pettenkoferstraße 9a, D-80336, Munich, Allemagne
10 Deutsches Zentrum für Infektionsforschung (DZIF), Ludwig-Maximilians-Universität, Munich, Allemagne
11 Division of Digestive and Liver Diseases, College of Physicians and Surgeons, Columbia University, New York, États-Unis
DOI : 10.1038/s41598-017-15567-3