Scientists at the Institut Pasteur, the CNRS and the University of Texas have been able to observe at atomic-level the effects of ethanol (the alcohol present in alcoholic beverages) on central nervous system receptors. They have identified five ethanol binding sites in a mutant of a bacterial analog of nicotinic receptors, and have determined how the binding of ethanol stimulates receptor activity. These findings can be directly extrapolated to human GABA receptors (the primary inhibitory neurotransmitters in the human brain), which are ethanol's main target in the central nervous system. This work is being published online on April 16, on the Nature Communications website. It paves the way for the synthesis of ethanol antagonist compounds that could limit the effect of alcohol on the brain.
Paris, april 16, 2013
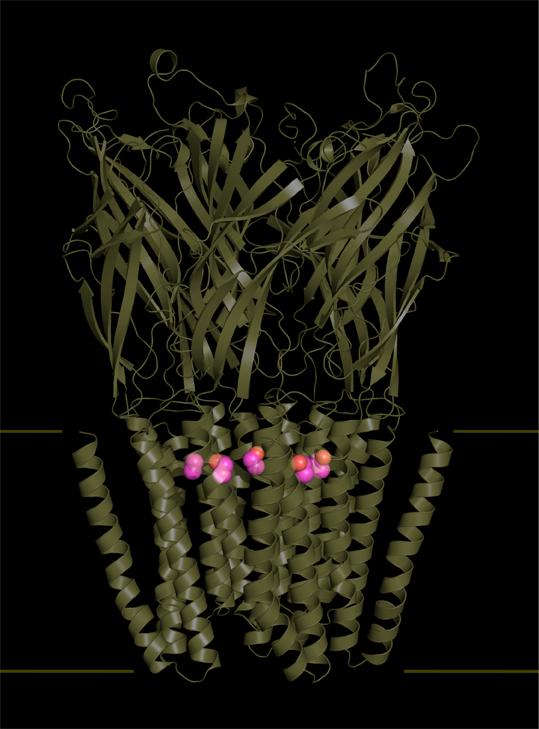
The teams led by Marc Delarue and Pierre-Jean Corringer, respective heads of the Structural Dynamics of Macromolecules unit and the Channel-Receptors group at the Institut Pasteur in Paris, along with researchers at the CNRS and scientists from the Waggoner Center for Alcohol and Addiction Research at the University of Texas have, for the first time, described the effects of ethanol at atomic level on its main target in the central nervous system.
The scientists have identified five ethanol binding sites in the structure of a bacterial homolog (from the model organism Gloeobacter violaceus) of nicotinic receptors and GABA receptors (also known as GABAA receptors). These receptors are particularly abundant on the neuron surface, and regulate the passage of nerve impulses via an ion channel that may be open or closed to either positively charged ions such as sodium ions (excitory neurotransmission, nicotinic receptors), or negatively charged ions such as chloride ions (inhibitory neurotransmission, GABA receptors).
Using X-ray crystallography, the scientists have made a breakthrough by determining the structure of ethanol in complex with its receptor, with a precision in the order of an Angstrom (one ten-billionth of a meter). In their study, the scientists compared the structure of the Gloeobacter violaceus receptor mutant to that of its human homolog. From this they observed that ethanol binding sites were perfectly preserved in human GABAA receptors (the main inhibitory neurotransmitter in the human brain), which are the preferred target of ethanol in the central nervous system. Determining this structure has enabled them to describe how ethanol fixation stabilizes the opening of the receptor channel, thereby disrupting cerebral functions by increasing the activity of inhibitory neurons.
This work opens new avenues for the development of antagonist compounds, which could be used as a substitute for ethanol, keeping receptor channels in the closed position. Such compounds could be used to limit the effects of alcohol consumption on the brain, as well as withdrawal effects in cases of dependence.
Sources
Structural basis for potentiation by alcohols and anesthetics in a ligand-gated ion channel, Nature Communications, April 16, 2013
Ludovic Sauguet (1,2,3,4), Rebecca J. Howard (5), Laurie Malherbe (1,2,3,4), Ui S. Lee (5), Pierre-Jean Corringer (3,4), R. Adron Harris (5) & Marc Delarue (1,2).
(1) Structural Dynamics of Macromolecules Unit, Institut Pasteur, F-75015 Paris, France.
(2) UMR 3528, CNRS, F-75015 Paris, France.
(3) Channel Receptors Group, Institut Pasteur, F-75015 Paris, France.
(4) URA 2182, CNRS, F-75015 Paris, France.
(5) Waggoner Center for Alcohol and Addiction Research, The University of Texas at Austin, Austin, Texas 78712.
Contact
Institut Pasteur Press Office
Nadine Peyrolo : + 33 (0)1 45 68 81 46
Jérémy Lescène : +33 (0)1 45 68 81 01
presse@pasteur.fr