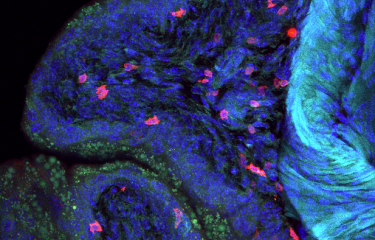
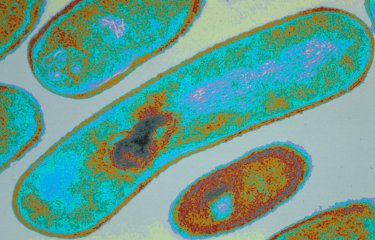
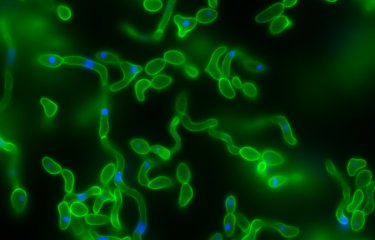
Candida albicans is a yeast that resides naturally in the gut, but its overgrowth can be fatal for immunocompromised patients. Scientists recently demonstrated that C. albicans overgrowth was controlled by β-lactamases, enzymes produced by certain bacteria in the gut microbiota.
Our gut contains thousands of billions of microorganisms, many more than the number of cells that make up our body. These microorganisms form what is known as the gut microbiota, which varies from one individual to the next.
Candida albicans is a yeast that can be found at low levels in the gut. But antibiotics can cause C. albicans to multiply dangerously and cross the intestinal wall. This can create conditions that are favorable for the development of systemic infection, known as systemic candidiasis, which is fatal in nearly half of all cases.
Measuring DNA to find yeast
To test this theory, scientists from the Institut Pasteur's Fungal Biology and Pathogenicity Unit called on 22 healthy volunteers. They were treated with cephalosporins, powerful β-lactam antibiotics that are often used in hospitals.
The scientists observed that fungi represented a small fraction of the species in the gut microbiota but that fungal populations varied significantly from one volunteer to the next. They used a very specific detection technique known as qPCR, which measures the quantity of DNA in a sample. "We discovered that C. albicans was present in nearly all the individuals, but sometimes at very low concentration, so much so that it would be undetectable using traditional culture methods – and this could give the impression that it was absent," says Christophe d'Enfert, head of the unit and co-author of the study.
The influence of antibiotics on C. albicans overgrowth
In other words, virtually anyone could develop candidiasis if their immune defenses are considerably weakened. So what are the effects of antibiotics? "Prior to the study, we thought that antibiotic treatments would have a similar effect in all the volunteers. But the reality was quite different: in some people, but not everyone, C. albicans proliferated considerably. We were able to correlate these differences with the level of β-lactamase activity in the microbiota of each volunteer," explains Margot Delavy, a scientist in the unit and co-author of the study. Some bacteria in the microbiota are capable of naturally producing β-lactamase, an enzyme responsible for resistance to certain antibiotics. "This could be a key factor in explaining individual variation in the risk of C. albicans overgrowth in the gut of vulnerable patients treated with antibiotics," continues Marie-Elisabeth Bougnoux, a scientist in the unit and co-author of the study.
It is too early to say whether these observations may lead to better prevention of C. albicans infection in vulnerable patients, but it undoubtedly opens new avenues for research.
This study comes under the Antimicrobial resistance priority scientific area in the Institut Pasteur's 2019-2023 Strategic Plan.
Source
A Clinical Study Provides the First Direct Evidence That Interindividual Variations in Fecal β-Lactamase Activity Affect the Gut Mycobiota Dynamics in Response to β-Lactam Antibiotics. MBio, 13(6). https://doi.org/10.1128/mbio.02880-22
Margot Delavy,a Charles Burdet,b,c Natacha Sertour,a Savannah Devente,d Jean-Denis Docquier,d Nathalie Grall,b Stevenn Volant,e Amine Ghozlane,e Xavier Duval,b,f France Mentré,b,c Christophe d’Enfert,a Marie-Elisabeth Bougnoux,a,g (2022).
aInstitut Pasteur, Université Paris Cité, INRAE USC2019, Fungal Biology and Pathogenicity Unit, Paris, France
bUniversité Paris Cité, IAME, INSERM, Paris, France
cAP-HP, Department of Epidemiology, Biostatistics and Clinical Research, Bichat Hospital, Paris, France
dUniversità di Siena, Dipartimento di Biotecnologie Mediche, Siena, Italy
eInstitut Pasteur, Université Paris Cité, Bioinformatics and Biostatistics Hub, Paris, France
fClinical Investigation Center, INSERM 1425, IAME, Bichat Hospital, Paris Public Hospital Network (AP-HP), Université Paris Cité, Paris, France
gParasitology-Mycology Unit, Clinical Microbiology Department, Necker-Enfants-Malades Hospital, Paris Public Hospital Network (AP-HP), Paris, France