Malaria and the globalization of disease
This 18 May 2022 is the centenary of the death of Alphonse Laveran, winner of the Nobel Prize for Medicine and discoverer of the protozoan responsible for malaria, which has caused hundreds of millions of deaths around the world since the dawn of humanity. Few diseases have left such a lasting legacy on the history and lives of humans as malaria. This parasitic disease, which tends to follow a seasonal pattern rather than giving rise to intermittent outbreaks, owes its name to the Italian mala aria, meaning "bad air" – reflecting the once widely held belief that stagnant water in marshes was capable of polluting the air and making it unsafe to breathe.
In ancient times, the parasite responsible for malaria (Plasmodium) was widespread in the Mediterranean basin. Hippocrates described bouts of fever recurring every three or four days (tertian or quartan fever), a rhythm that corresponds to the cycle of the parasite in the body. When the Roman Empire fell, the agricultural region around Rome – known as the "Roman Campagna" – was largely abandoned, further aggravating the malaria infestation and allowing Plasmodium to colonize northern Europe and then reach the New World via the triangular trade route.
Malaria is thought to have reached its peak in Europe between the 17th and 19th centuries, the period during which Alphonse Laveran discovered the parasite (see below). From the 20th century onwards, the disease declined in Europe because of several factors including the drying of wetlands, changing agricultural techniques and improved living standards. But if climate change causes temperatures to rise by 1 or 2 degrees, it could upset the fragile status quo that we have been enjoying ever since.
A heavy burden on the African continent
In 2020, the World Health Organization (WHO) estimated that more than half of the world's population was at risk of contracting malaria. That year there were 241 million cases and 627,000 deaths. Malaria infection is particularly severe in children under the age of 5, pregnant women, immunocompromised individuals and, to a lesser degree, people who have never been exposed to malaria and are likely to be: non-immunized migrants, mobile populations and travelers.
Although South-East Asia, Latin America and the Middle East are also affected, it is the African continent that pays the heaviest price: 95% of cases and 96% of deaths occur in sub-Saharan Africa. More recent data offer a glimmer of hope, however. Improved access to preventive measures, diagnostics and curative treatments over several years has ultimately paid off. According to WHO figures, the mortality rate fell by 63% between 2000 and 2019, although recent years have seen a weakening of health systems because of the COVID-19 epidemic. But we must remain on our guard at a time when we are witnessing growing resistance of parasites to antimalarial drugs and of mosquitoes to insecticides, and outbreaks have been observed outside the traditional endemic areas, including in South Africa, Costa Rica, Venezuela and Malaysia.
In France, the number of imported cases was estimated to be 2,895 in 2019. Ten deaths were reported, or a fatality rate of 0.35% for all cases and 2.5% for severe forms.

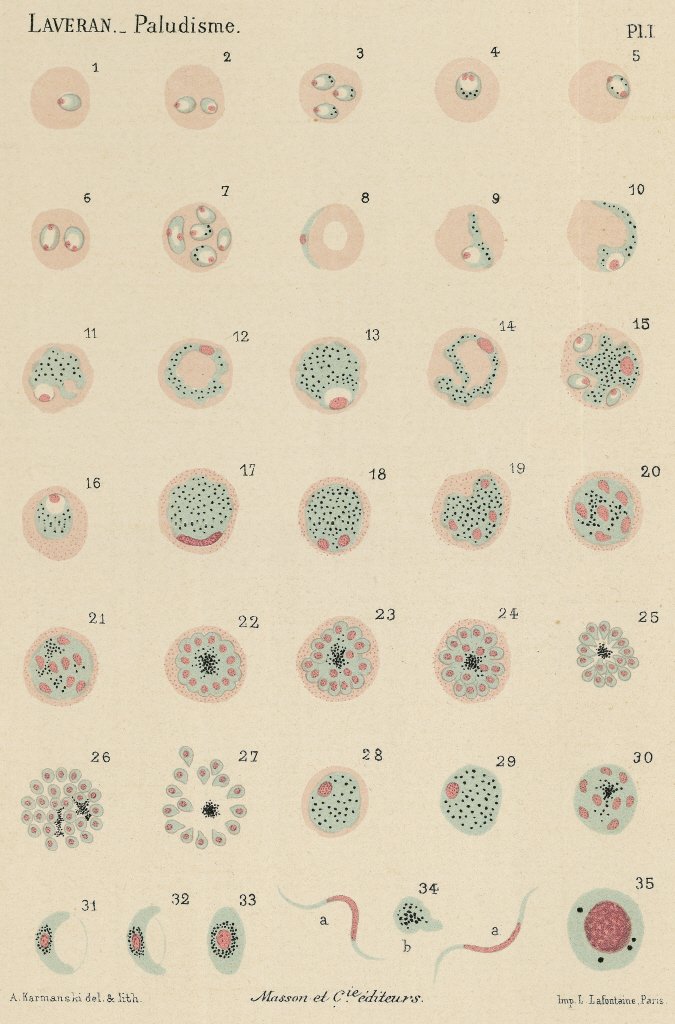 Work of Alphonse Laveran on the haematozoa, agent of malaria. Different aspects in fresh blood. Watercolour plate, 1880. |
A potentially fatal disease
Malaria is caused by a parasite of the genus Plasmodium. The disease is characterized by the sudden onset of high fever 8 to 30 days after the infectious bite. This can be accompanied by prodromal symptoms (early warning signs) including headache, general weakness, muscle pain, nausea, vomiting and a cough. Malaria attacks, with fever, shivering and hot and cold sweats, alternate with periods of respite. The periodic nature of malaria corresponds to the Plasmodium cycle, with attacks coinciding with the multiplication of the parasite and the moment at which it breaks out of red blood cells.
Parasites, mosquitoes and humans: a triangular relationship
Malaria is a vector-borne disease, which means that pathogens infect humans (or animals) via the intermediary of living organisms – usually hematophagous insects – that temporarily host the parasites. Plasmodium is transmitted by the bite of female Anopheles mosquitoes, of which there are more than 400 known species. Around 30 of them are likely to transmit the parasite. These mosquitoes need blood to reproduce and develop their eggs, which they then lay in water, where they hatch into larvae before becoming adults. Female mosquitoes become infected by biting infected individuals, and malaria is transmitted among humans via mosquitoes. Interhuman transmission occurs when the parasite is passed from mother to child via the placental barrier or by blood transfusion with infected blood.
The Plasmodium life cycle is complex. The parasites multiply by simple cell division (the asexual phase) in the liver and red blood cells of the human host. Some of these asexual parasites then develop into male and female sexual parasites. When the female Anopheles mosquito bites an infected individual, it ingests gametocytes1, which reproduce in its stomach and then travel to its salivary glands. The mosquito can then contaminate humans through a bite, thereby perpetuating the Plasmodium cycle.
 Immunofluorescence detection of Pf155/RESA protein in red blood cells infected with Plasmodium falciparum. |
1 Cells found in the blood of malaria-infected individuals which develops into a gamete (a reproductive cell) of the malaria parasite.
There are five parasite species that infect humans. Plasmodium falciparum is responsible for the most frequent and severe forms of malaria. It is the dominant species in Africa and can also be found in tropical areas in Latin America and Asia. If not treated within 24 hours, the disease it causes can prove fatal, especially since it leads to severe anemia with a dramatic fall in hemoglobin levels. Red blood cells infected with the parasite can also clump together and adhere to the linings of the small blood vessels that irrigate the brain, causing cerebral malaria, a syndrome with poor prognosis.
P. vivax is less harmful; it cohabits with P. falciparum in most endemic areas and is predominant in South America. The two other species are rarer and can cause late relapse: P. ovale, found in West Africa, can lead to attacks four to five years after the first symptoms, and P. malariae, which is distributed very unevenly worldwide, can cause relapses up to 20 years after an initial episode. Finally, human cases of infection with P. knowlesi, which generally infects monkeys, are regularly reported.
Prevention and treatment: growing resistance
There are two types of malaria treatment: preventive treatment, aimed at avoiding infection, and curative treatment to treat malaria attacks. Although there are several different preventive and curative drugs available to physicians, the parasite is becoming increasingly resistant to these treatments.

The Institut Pasteur has been actively involved for several decades in monitoring resistance of malaria parasites to antimalarial drugs. In malaria-endemic areas, it conducts clinical trials and epidemiological and genomic studies to assess whether treatment recommendations need to be updated to reflect changing levels of parasite susceptibility.
Didier MénardHead of the Malaria Genetics and Resistance Unit at the Institut Pasteur in Paris
Preventive treatment
The main preventive measures are not just based on drugs; they also involve methods to avoid bites from Anopheles mosquitoes. In recent years, the widespread use of vector control strategies has reduced the incidence of malaria in highly endemic areas. WHO and local health authorities advise two particularly effective forms of action: using insecticide-treated mosquito nets and spraying homes with residual insecticides (indoor residual spraying or IRS). For travelers, using mosquito nets, wearing long clothing treated with insecticide and applying insect repellent on exposed skin reduces the risk of being bitten.
Preventive treatment is crucial when traveling to a malaria-endemic area, especially for pregnant women and children, who are more at risk of severe malaria. Chemoprophylaxis can only be prescribed by a medical practitioner, since the nature and length of the treatment depends on several factors: the destination, individual characteristics (age, medical history, intolerance to drugs, etc.) and the duration of the trip. As well as the general advice available online, experts at the Institut Pasteur Medical Center offer specialist consultations to recommend suitable measures for each individual and each situation. Four main drugs are used, with the choice depending on whether the person is traveling to an area where resistance is detected. They are chloroquine (in chloroquine-sensitive areas, which are becoming increasingly rare), the atovaquone/proguanil combination, mefloquine (which is often not well tolerated) and doxycycline.
Chemoprophylaxis is not just for travelers; since 2012, WHO has recommended seasonal malaria chemoprevention in sub-Saharan Africa for pregnant women and children under the age of 5.
 The Anopheles Production and Infection Centre (CEPIA) is a platform dedicated to the mass production of Anopheles mosquitoes for research purposes. The CEPIA allows the study of the interactions between Plasmodium, the parasite responsible for malaria, and their mammalian or mosquito hosts. |
Curative treatment
Treatment begins once the diagnosis has been confirmed, generally by the identification of Plasmodium parasites following a microscopic examination of blood samples. Malaria rapid diagnostic tests have become more widely available over the past 20 years and can offer results in less than 30 minutes based on a finger-prick blood sample. Teams in the Pasteur Network regularly evaluate the performance of these rapid diagnostic tests.
Resistance of Plasmodium to traditional treatments such as chloroquine and the sulfadoxine/pyrimethamine combination became widespread in the 1950s and 1960s. In 2001, in light of the continuing emergence of resistance, WHO recommended using artemisinin-based combination therapies (ACTs) in South Asia and then Africa. Artemisinin is extracted from a plant that has been used for more than 2,000 years in China for its therapeutic properties. The recommendation is still applicable, since ACTs have proven effective despite the emergence of artemisinin resistance.
Scientists from the Institut Pasteur, involved in a WHO-supported project for molecular monitoring of resistance in Africa, recently identified the first signs of emergence of artemisinin-resistant K13 mutant parasites in Africa. The results describe significant proportions of parasites carrying the R561H mutation in two locations 100km apart (a prevalence of 7.4% in Masaka and 0.7% in Rukara). Whole-genome sequencing of these parasites indicates that the R561H mutants were selected from Rwandan parasite populations and that they had not spread from Asian parasites (from Thailand or Myanmar, where the R561H mutation has previously been observed). "These unexpected results contrast with previous scenarios in which the emergence of chloroquine- and pyrimethamine-resistant parasites in Africa was caused by the spread of resistant parasites from South-East Asia. It was thought that a similar scenario would apply for the emergence of artemisinin-resistant parasites in Africa," explains Didier Ménard, Head of the Malaria Genetics and Resistance Unit at the Institut Pasteur in Paris. The fact that this resistant strain has spread between several places in Rwanda and its ability to resist artemisinin in vitro have major public health implications. In the absence of effective measures to contain the spread of resistant parasites in Rwanda and neighboring countries, there is a risk that over time they will acquire the ability to resist the partner drugs used in ACTs. This would mean that the only available treatments would become ineffective, as has occurred in South-East Asia. A model of this scenario, in which no measures are taken, recently predicted that the inefficacy of ACTs in Africa could be responsible for 78 million additional cases and 116,000 additional deaths over a five-year period.
Epidemiology of resistance
The growing resistance of the parasite to available treatments represents a major public health problem. Regular monitoring is required to assess the sensitivity of Plasmodium and how it is evolving in different world regions. WHO is committed to this task, and the Pasteur Network also plays an active part, especially the members in malaria-endemic countries like Cambodia, Madagascar, Senegal, Côte d'Ivoire, Niger and Central African Republic. The Institut Pasteur de la Guyane is the National Reference Center for Drug Resistance in Malaria for the Antilles-French Guiana Region. The team led by Lise Musset is investigating parasites and their resistance by testing the sensitivity of collected samples to eight molecules used in therapeutics and identifying resistance genes. The team has demonstrated that a mutation in the pvmdr1 gene enables the Plasmodium vivax strains circulating in French Guiana to resist chloroquine.
 Setting up a mosquito trap in the commune of Matoury, as part of studies on malaria transmission (entomological mission in Maripasoula, Guyana, March 2016). |
Obstacles to vaccination
A first malaria vaccine, RTS,S/ASO1, has been available since 2016. It has received a positive opinion from the European Medicines Agency and has been authorized by WHO for children on the African continent. But its efficacy is limited and the four doses required do not confer lasting immunity. On October 6, 2021, WHO recommended using the RTS,S vaccine to prevent P. falciparum malaria in children living in regions with moderate to high transmission.
The main obstacle for the Plasmodium vaccine is that the parasite evades the immune system. A first attack does not lead to the production of antibodies or elicit a memory response to infection. Only people with chronic malaria benefit from some degree of protection. To develop the vaccine, scientists fused an antigen from the parasite with the hepatitis B antigen, with the latter responsible for triggering the immune response.
In the Institut Pasteur's Biology of Host-Parasite Interactions Unit, led by Artur Scherf, Salaheddine Mécheri has developed an innovative vaccine approach for malaria. Previous research by the group demonstrated that the early production of cytokine IL-6 (a protein involved in immunity) in the host liver led to the acquisition of long-term immune protection. The scientists explored an approach in which mice were inoculated with a parasite that itself encodes the mouse IL-6 gene. Immunizing mice with transgenic parasites elicited long-lasting protective immunity against subsequent attacks by infectious parasites. The study demonstrated that the IL-6 encoded by the transgenic parasite impaired infection with wild-type Plasmodium parasites, which remained stalled at the liver stage. This therefore represents a novel vaccine strategy to confer protective immunity against malaria. This novel vaccine tool is now being extended to the Plasmodium falciparum parasite, the strain that infects humans.
Chetan Chitnis, Head of the Malaria Parasite Biology and Vaccines Unit, is developing a vaccine for Plasmodium vivax, the second most prevalent Plasmodium species, which is responsible for considerable morbidity. P. vivax has a unique characteristic: it depends on an interaction with the Duffy antigen, located on human red blood cells, to infect these cells. The team led by Chetan Chitnis has identified the binding domain of the P. vivax Duffy binding protein (PvDBP) and is developing a vaccine based on this functional domain. Antibodies targeting PvDBP inhibit the interaction of P. vivax with red blood cells and may prevent blood-stage infection. A vaccine based on this strategy is currently in early-stage clinical trials in collaboration with the Jenner Institute at the University of Oxford and the International Center for Genetic Engineering and Biotechnology (ICGEB) in New Delhi.
The Malaria Infection and Immunity Unit is investigating sporozoites, the cells in the malaria parasite that infect new hosts by leaving the mosquito and penetrating the liver, where they multiply. The team recently discovered how cytotoxic antibodies directed against the sporozoites' major surface protein (the circumsporozoite protein or CSP), which is the antigenic basis for the first malaria vaccine, can effectively block infection in host tissues. By identifying protective antigens and combining them with the CSP, a multi-antigen vaccine was developed with highly protective efficacy in a preclinical model.
Entomology to tackle vector-borne diseases
With more than 3 million insect species described to date, entomologists – scientists who study insects – are facing a mammoth task. Some species can transmit infectious agents to humans and are vectors of disease. These are insects that bite and feast on blood meals. This troublesome bestiary includes the following, in no specific order: ticks (Lyme disease), flies (sleeping sickness or trypanosomiasis caused by the tsetse fly), blackflies (onchocerciasis or "river blindness"), fleas (plague), triatomine or "kissing" bugs (Chagas disease) and, of course, mosquitoes, which are vectors of infectious and parasitic diseases including malaria, chikungunya, dengue, Zika and also Rift Valley fever, yellow fever, West Nile fever, Japanese encephalitis and lymphatic filariasis. Mosquitoes are public enemy number one, capable of transmitting more than a hundred diseases.
Understanding mosquitoes' physiology and ability to adapt is a key priority in tackling vector-borne diseases, especially when it comes to those mosquitoes that transmit the dengue, Zika and chikungunya viruses (known as arboviruses). At the Institut Pasteur in Paris, the Arboviruses and Insect Vectors Unit led by Anna-Bella Failloux studies interactions between mosquitoes, viruses and the environment. It identifies factors that encourage the emergence of pathogens responsible for human disease outbreaks, factors related to the interactions between arboviruses and their vectors, and also environmental and/or climate changes that are affecting the organization of mosquito populations. The unit has a facility for rearing mosquitoes which houses the species that are most harmful for humans, and a BSL3 laboratory where animal models can be experimentally infected. Anna-Bella Failloux and her team work closely with the Pasteur Network member institutes.
 Merozoite stage of Plasmodium falciparum, an infective form that will invade the red blood cell. |
The Institut Pasteur de la Guyane has launched a large-scale epidemiology survey (EPI-ARBO) among the population to establish a detailed picture of the impact of arboviruses in French Guiana. Since 2014 the institute has had an insect vector research facility, the Emile Abonnenc Amazonian Vectopole, named after the first entomologist at the Institut Pasteur de la Guyane. In this insectarium, entomologists classify mosquitoes which have a cycle of approximately 700 generations each year and are therefore constantly evolving and producing new species. No fewer than 250 of these species, of the more than 3,500 known species worldwide, have been recorded in this South American region to date. A laboratory monitors how resistance to insecticides is evolving among these arthropods. Lastly, like in Paris, a BSL3 laboratory performs experimental infections. In Cambodia, a medical entomology facility led by Sébastien Boyer and supervised by Didier Fontenille, director of the local Institut Pasteur, was established in 2015. As well as malaria, dengue, Zika and chikungunya, Japanese encephalitis is also the focus of detailed research. Other Pasteur Network member institutes, including those in Bangui and Dakar, are actively involved in monitoring the mosquitoes that are vectors of arboviruses via an unparalleled international network.
 Red blood cell parasitized by Plasmodium falciparum |
Hereditary anemias, mainly sickle cell anemia and thalassemia, are genetic hemoglobin disorders. As early as the 1920s, physicians wondered whether there may be a link between these conditions and malaria. They had noticed that the diseases had the same geographical distribution, in both sub-Saharan Africa and the Mediterranean basin. And they needed to find a reason why the harmful genes of these diseases were so widespread. Finally, Italian specialists identified that in Sardinia, then a highly endemic area for malaria, thalassemia was frequent in coastal regions and virtually absent from mountainous areas. Population geneticist John B. S. Haldane came up with a solution to this enigma: individuals with a single copy of the sickle cell gene (heterozygotes) were more resistant to malaria than those with normal hemoglobin. In heterozygotes, thalassemia represents a selective advantage in malaria endemic areas, since the defective red blood cells interfere with the parasite's life cycle.
But hereditary anemia is rare in areas where there is no malaria, like mountainous regions where mosquitoes disappear above a certain altitude. This hypothesis was confirmed by Anthony C. Allison in 1954: he showed that the levels of Plasmodium in the blood of people living on the banks of Lake Victoria were lower in those with sickle cell trait (heterozygous carriers).
An inherited genetic disorder can therefore interfere with a vector-borne parasitic disease which has itself played a part in the selection of human populations.