Researchers at the CNRS and the Institut Pasteur have discovered the mechanism by which nutrients enter a bacterium. Thanks to cutting-edge technologies, they have completed the puzzle of a sequence of events in the bacterial envelope. They deciphered the workings of a "molecular motor" that brings nutrients into bacteria, and explain how energy circulates to bring them in. The proof is in the pictures.
Bacteria have a fundamental need for certain rare nutrients such as metals, vitamins and certain sugars. Without these nutrients, the bacterium is deficient, which impairs functions such as host infection. Nutrients penetrate the outer bacterial membrane with the help of surface receptors (proteins) and energy from molecular motors on the inner membrane side.
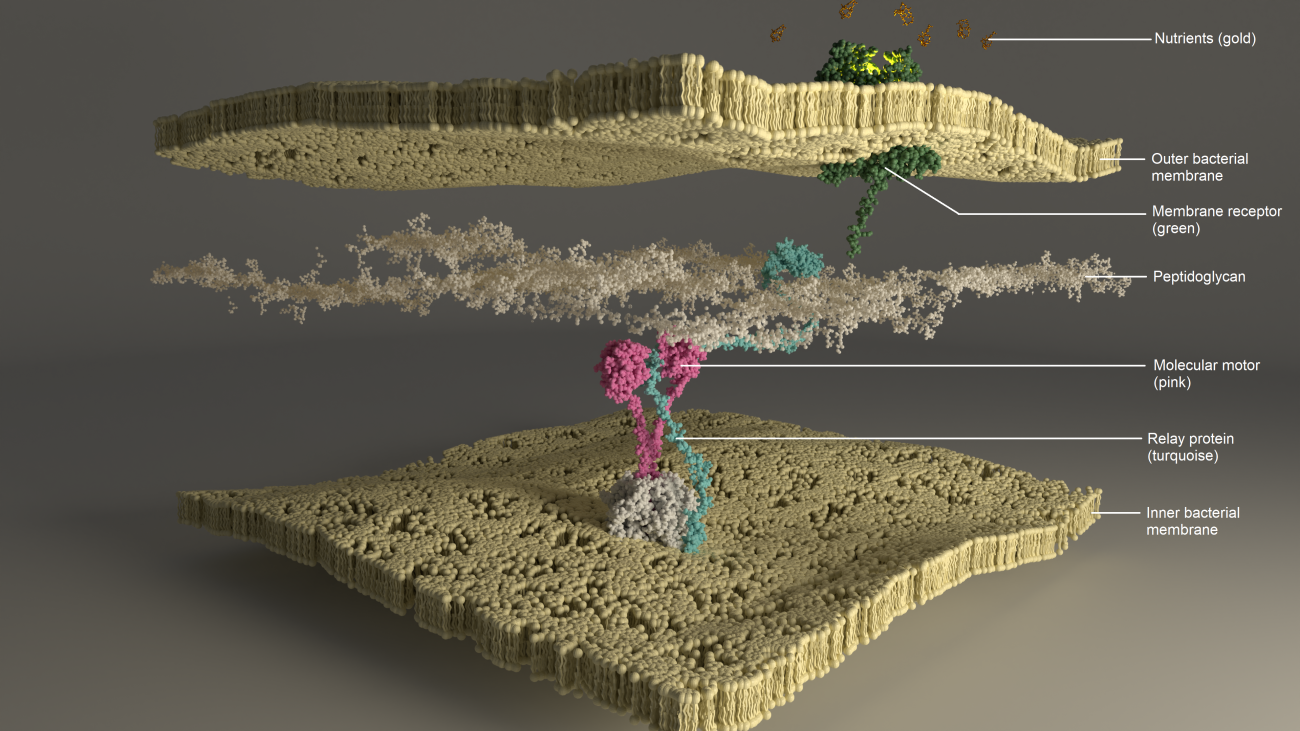
Previously, the full structure of these proteins, also known as molecular motors, was unknown (although they were discovered as early as the 1970s, we did not have the technology to probe the structure as well as we can today), nor was it known how energy was transferred across the envelope. This mechanism was all the more mysterious in that the molecular motors were distant from the receptor. The architecture of the system, which traverses the bacterial envelope, and the dynamics of the various components and their interactions, made studying them highly complex.
By combining structural biology and microbiology, Nadia Izadi-Pruneyre's Bacterial Transmembrane Systems team (Institut Pasteur/CNRS*) succeeded in completing the atomic structure of the molecular motor. The team has shown for the first time that the central part of the motor has two states, open or closed. This characteristic gives them a particular dynamic, on a time scale ranging from microseconds to milliseconds, enabling them to transfer energy.
"The interaction of nutrients outside the membrane with the membrane receptor triggers a whole process creating protein dynamics. At the end of this sequence, a channel opens up and nutrients can enter," explains Nadia. Functional tests revealed that these dynamics and the transition between the two states are essential for nutrient import and bacterial survival. "Once we understood this energy transfer, we observed that only the open state was capable of interacting with the other components of the membrane envelope and the system," explains explique Maximilan Zinke, first author of this study.
Three-dimensional animation of energy transfer by the molecular motor to move nutrients into the bacterial membrane © Institut Pasteur/Maximilan Zinke
Nuclear magnetic resonance, or NMR, spectroscopy was crucial to this discovery. Its contribution lies in its unique ability to provide atomic-scale structural and dynamic information under physiological conditions. Combined with other technologies dedicated to structural biology, such as the recently developed electron cryomicroscopy and X-ray crystallography, it is essential for understanding complex, dynamic machineries such as bacterial molecular motors.
These molecular motors are highly conserved in bacteria, providing energy for a variety of vital functions. "We assume that this mechanism is widespread" in all bacteria. This discovery opens up new prospects in the fight against antibiotic resistance. "We could envisage keeping these mills active all the time, using inhibitors, which would deplete and block the bacteria," says Nadia. Some metals are a source of competition between the host's immune system and bacteria. In this case, the host organism sets up a whole system to sequester certain metals, such as iron, so that the bacteria can't use them.
* In collaboration with the X-ray Crystallography platform and Ivo Boneca at the Institut Pasteur, and Philippe Delepelaire at IBPC-CNRS.
This study is part of the priority scientific area Antimicrobial Resistance of the Institut Pasteur's strategic plan for 2019-2023.
Source:
Ton motor conformational switch and peptidoglycan role in bacterial nutrient uptake, Nature Communications, December 20, 2023


