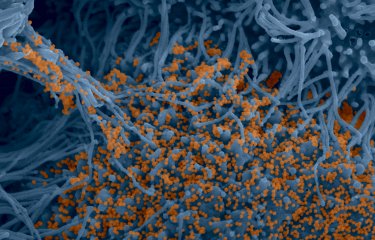
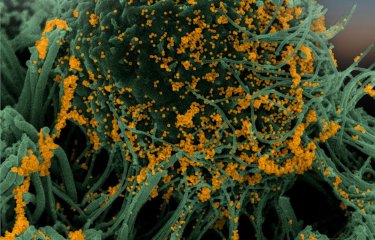
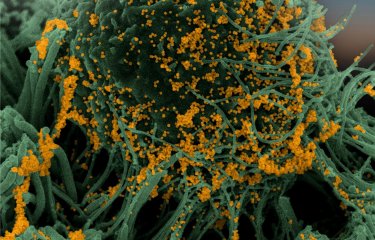
Bats are reservoir species for numerous viruses including coronaviruses. Given that they do not appear to be affected by diseases transmitted by these viruses, there is potential benefit in understanding how their immune system regulates infection. Scientists at the Institut Pasteur and the CNRS studied SARS-CoV-2 replication in bat cells, which involved using real-time imaging techniques for the first time. They demonstrated the existence of species-specific and cell-specific immune responses. These findings were published in The Journal of Virology on July 5, 2022.
Bats are reservoir species for numerous viruses. Unlike humans and other mammals, they are able to carry certain viruses without becoming ill following infection. Bats' immune systems therefore appear capable of controlling certain viral infections effectively. Scientists at the Institut Pasteur and the CNRS examined SARS-CoV-2 replication in bat cells to understand how these animals' cells respond to viral infection.
Their first task was to develop cellular models. This entailed gathering cell lines previously generated by other laboratories and producing new ones. Among the contributor labs were those involved in the collaborator network of the National Reference Center for Rabies at the Institut Pasteur. Bat cell cultures were derived from various bat species and various organs (brain, skin, digestive tract). "These animals are the world's second largest group of mammals with nearly 1,400 different species; it's important to have the widest possible range of bat cellular models so that we can compare and extrapolate our results. These cellular models are, moreover, essential for any research on bats," explains Laurent Dacheux, a scientist in the Lyssavirus, Epidemiology and Neuropathology Unit, Deputy Director of the National Reference Center for Rabies at the Institut Pasteur, and co-last author of the study.
The scientific teams adapted these cell cultures by modifying them to express the viral receptor, human ACE2 protein. This enabled them to develop a collection of cell lines relevant to research on SARS-CoV-2 and other related viruses.
The cell lines were then infected with SARS-CoV-2 and observed using different microscopy techniques including real-time optical microscopy. "This is the first time it has been possible to visualize SARS-CoV-2 replication in living bat cells," comments Nolwenn Jouvenet, Research Director at the CNRS, Head of the Virus Sensing and Signaling Unit at the Institut Pasteur, and co-last author of the study. Through time-lapse microscopy, scientists were able to determine the speed at which cells were infected. Cells from some cell lines died quickly, with syncytia[1] forming rapidly.
Video of cells derived from greater mouse brain (Myotis myotis) infected with SARS-CoV-2 (right) or uninfected (left). The cells are cultured in a medium containing propidium iodide as a cell death marker. Cells were photographed every 15 minutes for 48 hours. © Delphine Planas, Sophie Aicher, Nolwenn Jouvenet (Institut Pasteur)
"By installing a microscope in a BSL3 laboratory in the midst of the COVID-19 pandemic, we were able to visualize the consequences of SARS-CoV-2 infection on cell functioning in real time," adds Olivier Schwartz, Head of the Virus and Immunity Unit at the Institut Pasteur[2] and co-author of the study.
Using these ACE2-expressing bat cells and a wide range of virology, biochemistry, and optical and electron imaging techniques, the scientists demonstrated that viral replication was controlled over time in some bat cell lines, and that this control was linked to a powerful, immediate immune response that was impossible for the virus to evade.
Viral replication was blocked at different stages depending on the cell line. In some cell lines, the virus was unable to replicate even after penetrating cells. In other lines, although the virus was able to replicate, no viral particles were released into the extracellular environment. On the whole, these findings suggest the existence of species-specific molecular barriers affecting coronavirus replication in bat cells.
Electron microscopy image showing the accumulation of viral particles at the surface of cells derived from the brain of a bat (species: Myotis myotis).
© Philippe Roingeard (University of Tours), Sophie-Marie Aicher, Nolwenn Jouvenet (Institut Pasteur/CNRS)
"Our task is now to identify and characterize proteins induced by the immune response in bats that SARS-CoV-2 is unable to block," adds Nolwenn Jouvenet. To achieve this goal, the scientists will use the large collection of cell lines they have developed and high-throughput transcriptomic techniques to identify immunity genes with antiviral properties. "We still don't know why bats' immune system is so effective at fighting off coronaviruses. The challenge lies in improving our understanding of how this animal reservoir is able to carry viruses that affect global health," she concludes.
A 2021 Nikon "Small World in Motion" award-winning video.
On August 16, 2021, scientists from the Virus Sensing and Signaling Unit and the Virus and Immunity Unit at the Institut Pasteur received an honorable mention at Nikon's Small World in Motion video competition. Their time-lapse video shows bat cells being infected by SARS-CoV-2 for the very first time. One image was captured every 15 minutes for 48 hours, with a fluorescent red molecule used to visualize cell death. This red marker enters cells while they are dying and is used to quantify cell death.
Thanks to the "Coronavirus Task Force" set up on January 23, 2020, it was possible to install the microscope in a BSL3 laboratory. The Task Force is composed of scientific experts from several disciplines and support staff from the Institut Pasteur's technical departments to ensure that scientific projects can be carried out as effectively as possible.
Link to the video
[1] Syncytia are giant cells composed of tens of cells.
[2] This is named the "Virology Unit" at the CNRS (CNRS/Institut Pasteur).
Source
Species-specific molecular barriers to SARS-CoV-2 replication in bat cells, Journal of Virology, July 5, 2022
Sophie-Marie Aicher1, Felix Streicher1, Maxime Chazal1, Delphine Planas2,3, Dongsheng Luo4, Julian Buchrieser2, Monika Nemcova5, Veronika Seidlova5, Jan Zukal6, Jordi Serra-Cobo7,8, Dominique Pontier9,10, Bertrand Pain11, Gert Zimmer12, Olivier Schwartz2,3, Philippe Roingeard13, Jiri Pikula5, Laurent Dacheux4*, Nolwenn Jouvenet1*
1 Institut Pasteur, Université de Paris Cité, CNRS UMR 3569, Virus sensing and signaling Unit, Paris, France
2 Institut Pasteur, Université de Paris Cité, CNRS UMR 3569, Virus and Immunity Unit, Paris, France
3 Vaccine Research Institute, Créteil, France
4 Institut Pasteur, Université de Paris Cité, Lyssavirus Epidemiology and Neuropathology Unit, Paris, France
5 Department of Ecology and Diseases of Zoo Animals, Game, Fish and Bees, University of Veterinary Sciences Brno, Brno, Czech Republic
6 Institute of Vertebrate Biology of the Czech Academy of Sciences Brno, Czech Republic
7 Institut de Recerca de la Biodiversitat (IRBio), Faculty of Biology, Universitat de Barcelona, Barcelona, Spain
8 Departament de Biologia Evolutiva, Ecologia i Ciències Ambientals, Facultat de Biologia, Universitat de Barcelona
9 Université de Lyon, LabEx Ecofect, Lyon, France
10 Université Lyon 1, CNRS, Laboratoire de Biométrie et Biologie Evolutive UMR5558, Villeurbanne, France
11 University of Lyon, Université Lyon 1, INSERM, INRAE, Stem Cell and Brain Research Institute, U1208, USC1361, Bron, France.
12 Institute of Virology and Immunology, Bern & Mittelhäusern, Switzerland, and Department of Infectious Diseases and Pathobiology, Vetsuisse Faculty, University of Bern, Bern, Switzerland
13 INSERM U1259 MAVIVH and Plateforme IBiSA de Microscopie Electronique, Faculté de Médecine, Université de Tours, Tours, France
*co-corresponding/co-senior authors