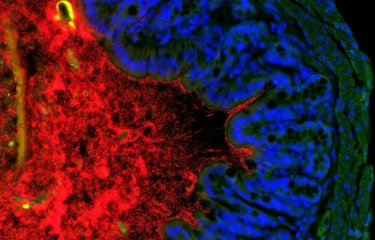
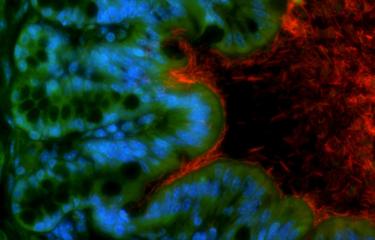
Scientists from the Institut Pasteur, in collaboration with the Institut Gustave-Roussy, have identified two bacterial species naturally present in the body, Enterococcus hirae and Barnesiella intestinihominis, which enhance the effect of cyclophosphamide, a common chemotherapy treatment. The scientists had already proven the role of the microbiota in the efficacy of chemotherapy. This time, they characterized two bacteria responsible for this action.
Cyclophosphamide is a chemotherapy molecule frequently used to treat various forms of cancer (including lung, breast and ovarian cancer). It is on the model list of "Essential Medicines" published by the World Health Organization (WHO), which contains a selection of drugs that meet the health care needs of the population. We know that one of the common side effects of cyclophosphamide is inflammation of the intestinal mucosa. This weakens the intestinal barrier and allows bacteria in the intestine (the microbiota) to cross this barrier and pass into the bloodstream and the lymph nodes. The body is generally able to identify invasive bacteria and eliminate them, but patients receiving chemotherapy are often also given antibiotics to avoid infection.
An irony of this intestinal inflammation is that our intestinal bacteria strengthen the therapeutic effect of chemotherapy (as discovered by the Institut Pasteur and the Institut Gustave-Roussy in 2013). "The efficacy of chemotherapy partly depends on intestinal bacteria known as 'oncomicrobiotics'," explained Ivo Gomperts Boneca, Head of the Institut Pasteur's Biology and Genetics of the Bacterial Cell Wall laboratory.
It was not previously known which of the billions of bacteria that make up the microbiota crossed the intestinal barrier and helped destroy tumors. In the study that was published in early October, the same scientists from the Institut Pasteur in Paris, the Institut Pasteur in Lille and the Institut Gustave-Roussy identified two bacterial species, Enterococcus hirae and Barnesiella intestinihominis, which play a role during cyclophosphamide treatment.
"We observed in mice that, if the microbial ecosystem is destabilized, as it is during chemotherapy, Enterococcus hirae bacteria alter the environment near the tumor and reinstate an effective response from T cells [immune cells] against the tumor," continued Ivo Gomperts Boneca. This action mechanism had previously remained a mystery. The bacteria therefore help prevent the progression of the tumor. Moreover, the scientists also proved that Barnesiella intestinihominis is involved in inducing a memory immune response to tumors.
So Enterococcus hirae strengthens the body's natural immune response to the tumor, and although this beneficial effect of E. hirae is only temporary, Barnesiella maintains the response over the long term.

A photo of Enterococcus hirae produced at the Institut Pasteur's Ultrapole. © Chantal Ecobichon / Institut Pasteur
Another of the scientists' 2016 findings was that in a cohort of 16 advanced lung and ovarian cancer patients treated with cyclophosphamide, those with Enterococcus hirae and Barnesiella intestinihominis bacteria in their microbiota responded more favorably to treatment.
Enterococcus hirae and Barnesiella intestinihominis are therefore bacteria in our bodies that play a valuable role in improving the efficacy of one of the most common chemotherapy treatments.
"There are probably other useful bacteria in our microbiota," continued Ivo Gomperts Boneca. "We observed that not all the patients receiving chemotherapy reacted to treatment in the same way. This can be explained by the fact that all individuals have their own microbiota that is not necessarily composed of the same bacteria."
This observation gives rise to two questions: could these bacteria be "given" to those patients who are lacking them? Or rather than the bacteria themselves, could the patients be given the components of the bacteria involved in this anti-cancer activity? "We are exploring the latter option," said the scientist, "because injecting the bacteria themselves into the microbiota could cause infection. We are investigating how to characterize the molecules responsible for this activity so that we can mimic the action of the bacteria."
This approach confirms the possibility of personalized medicine for cancer treatment, to help patients who currently respond poorly to chemotherapy.
Source
Enterococcus hirae and Barnesiella intestinihominis involved in cyclophosphamide-induced therapeutic immunomodulatory effects, Immunity, 18 octobre.
Romain Daillère1,2,3, Marie Vétizou1,2,3, Nadine Waldschmitt4, Takahiro Yamazaki1,2, Christophe Isnard5,6, Vichnou Poirier-Colame1,2,3, Connie P. M. Duong1,2,7, Caroline Flament1,2,7, Patricia Lepage8, Maria Paula Roberti1,2,7, Bertrand Routy1,2,3, Nicolas Jacquelot1,2,3, Lionel Apetoh9,10,11, Sonia Becharef1,2,7, Sylvie Rusakiewicz1,2,7, Philippe Langella8, Harry Sokol8,12,13, Guido Kroemer14,15,16,17,18,19, David Enot1,15, Antoine Roux1,2,3,18, Alexander Eggermont1,3, Eric Tartour20,21, Ludger Johannes22,23,24, Paul-Louis Woerther25, Elisabeth Chachaty25, Jean-Charles Soria1,3, Benjamin Besse1,3, Encouse Golden26, Silvia Formenti26, Magdalena Plebanski27, Mutsa Madondo27, Philip Rosenstiel28, Didier Raoult29, Vincent Cattoir*5,6,30, Ivo Gomperts Boneca*31, Mathias Chamaillard*4 and Laurence Zitvogel1,2,3,7.
1 Institut de Cancérologie Gustave Roussy Cancer Campus (GRCC), 114 rue Edouard Vaillant, Villejuif, 94805, France ;
2 Institut National de la Santé Et de la Recherche Medicale (INSERM), U1015, GRCC, Villejuif, 94805, France ;
3 University of Paris-Saclay, Kremlin Bicêtre, 94270, France ;
4 Institut Pasteur de Lille, Center for Infection and Immunity of Lille, Lille, 59800, France ;
5 Université de Caen Basse-Normandie, EA4655 U2RM (Équipe Antibio-Résistance), Caen, 14033, France ;
6 CHU de Caen, Service de Microbiologie, Caen, 14033, France ;
7 Center of Clinical Investigations in Biotherapies of Cancer (CICBT) 1428, Villejuif, 94805, France ;
8 Micalis Institute, INRA, AgroParisTech, Université Paris-Saclay, 78350 Jouy-en-Josas, France ;
9 Lipids, Nutrition, Cancer, INSERM, U866, Dijon, 21078, France ;
10 Department of Medicine, Université de Bourgogne Franche-Comté, Dijon, 21078, France ;
11 Department of Oncology, Centre Georges François Leclerc, Dijon, 21000, France ;
12 AVENIR Team Gut Microbiota and Immunity, ERL, INSERM U 1157/UMR 7203, Faculté de Médecine, Saint-Antoine, Université Pierre et Marie Curie (UPMC), Paris, 75012, France ;
13 Service de Gastroentérologie, Hôpital Saint-Antoine, Assistance Publique—Hôpitaux de Paris (APHP), Paris, 75012, France ;
14 INSERM U848, 94805 Villejuif, France ;
15 Metabolomics Platform, Institut Gustave Roussy, Villejuif, 94805, France ;
16 Equipe 11 labellisée Ligue contre le Cancer, Centre de Recherche des Cordeliers, INSERM U 1138, Paris, 75006, France ;
17 Pôle de Biologie, Hôpital Européen Georges Pompidou, AP-HP, Paris, 75015, France ;
18 Université Paris Descartes, Sorbonne Paris Cité, Paris, 75006, France ;
19 Karolinska Institute, Department of Women's and Children's Health, Karolinska University Hospital, Stockholm, 17176, Sweden ;
20 INSERM U970, Paris Cardiovascular Research Center, Université Paris-Descartes, Sorbonne Paris Cité, Paris, 75015, France ;
21 Service d’immunologie biologique, Hôpital Européen Georges Pompidou, Paris, 75015 France ;
22 INSERM U1143, 75005 Paris, France ;
23 Institut Curie, PSL Research University, Endocytic Trafficking and Therapeutic Delivery group, Paris, 75248, France ;
24 CNRS UMR 3666, Paris, 75005, France ;
25 Service de microbiologie, GRCC, Villejuif, 94805, France ;
26 Department of Radiation Oncology, Weill Cornell Medicine, New York, NY, USA ;
27 Department of Immunology and Pathology, Monash University, Alfred Hospital Precinct, Melbourne, Prahran, Victoria 3181, Australia ;
28 Institute of Clinical Molecular Biology, Christian-Albrechts-University and University Hospital Schleswig-Holstein, Campus Kiel, 24105 Kiel, Germany ;
29 Aix Marseille Université, URMITE (Unité de Recherche sur les Maladies Infectieuses et Tropicales Emergentes), UMR 7278, INSERM 1095, IRD 198, Faculté de Médecine, Marseille 13005, France ;
30 CNR de la Résistance aux Antibiotiques, Laboratoire Associé Entérocoques, Caen, 14033, France ;
31 Institut Pasteur, Unit Biology and Genetics of the bacterial Cell Wall, Paris, 75015, France.
*All three authors equally contributed to this work
Mis à jour le 18/10/2016