Alzheimer's disease is a progressive neurodegenerative disorder that mainly affects memory, but also other cognitive functions linked to knowledge and involving language, logical thought and learning capacity. This disease generally leads to loss of independence for sufferers. It is one of the leading causes of disability and dependency in elderly people. There is currently no cure for the disease, and there are still many unanswered questions about the mechanisms of this disease.
In Alzheimer's disease, the tau protein, which stabilizes neuron structure, is altered. This causes a loss of neuronal architecture, neurofibrillary degeneration and the death of nerve cells (read the Institut Pasteur Alzheimer’s disease fact sheet). The misfolded tau proteins aggregate and form neurofibrillary tangles, which accumulate progressively inside the neurons. Neurofibrillary tangles can seed abnormal conformations on normal tau proteins, in a prion-like manner, initiating a self-amplifying cascade, and can spread from their initial production site to other areas in the brain. This is how the disease pathology eventually spreads to all parts of the brain. However, the underlying mechanism is still unclear, as the fate and behaviour of endogenously formed aggregates has not been assessed.
Real time observation of endogenously expressed Tau and its conversion into aggregates
The Membrane Traffic and Pathogenesis Unit at the Institut Pasteur (Paris), with GlaxoSmithKline and the Brain Institute of the University of Texas Southwestern Medical Center, investigated this mechanism. “We established a neuronal reporter cell system, allowing to record in real time the conversion of endogenously expressed Tau RD domain from soluble state to aggregates upon addition of external fibrils, either synthetically produced or from Alzheimer’s disease patient brain extracts. We showed that seeding is not rate-limiting and follows the same kinetic with both sources of fibrils,” explains Chiara Zurzolo, head of the Membrane Traffic and Pathogenesis Unit.
Endogenous Tau aggregates block their own autophagic degradation and propagate though TNTs
“Further, the endogenously-formed aggregates are recognized as autophagic cargoes, but fail to be transferred to lysosomes for degradation as the autophagy flux is partly blocked.” This suggests that endogenously formed Tau aggregates block their own degradation through the autophagic pathway. Both endogenous and exogenous aggregates are transmitted between cells in a contact dependent manner, transferring through tunnelling nanotubes (TNT) to neighbouring cells.
These data extend previous research by the same team (Membrane Traffic and Pathogenesis Unit at the Institut Pasteur) showing that TNTs are involved in the intercellular spreading of pathogenic amyloid proteins involved in Alzheimer’s and Parkinson’s disease, highlighting that similar pathways, that might be targeted to develop novel therapeutics, are involved in the progression of different neurodegenerative diseases.
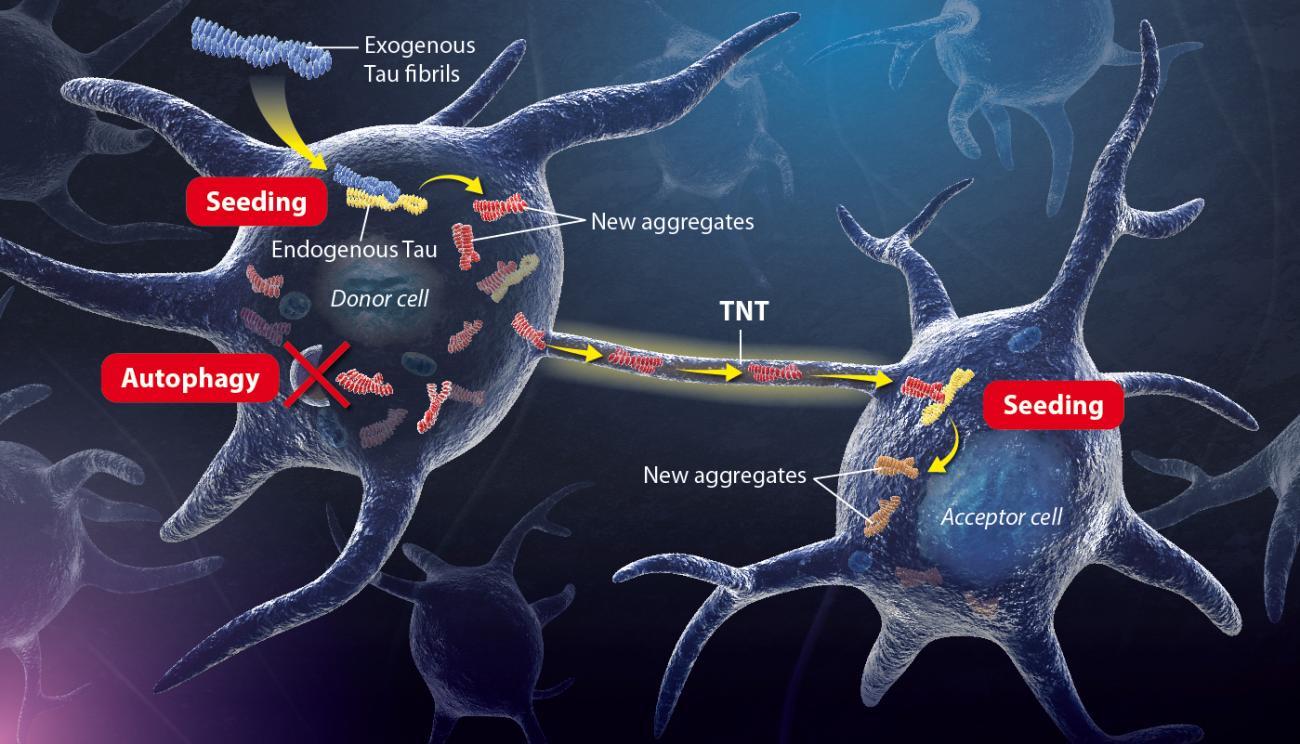
Illustration of the mechanism uncovered by Chiara Zurzolo’s Team. Copyright: Institut Pasteur / P. Marseaud.
Exogenous tau fibrils - aggregated exogenous Tau proteins enter the neuron cell and accumulate progressively.
Seeding – The exogenous tau fibrils seed abnormal conformations on normal tau proteins, initiating a self-amplifying cascade.
Autophagy – endogenous tau aggregates block their own autophagic degradation.
Transfer- Neurofibrillary tangles spread to neighbouring cells through TNTs and trigger the seeding process in naïve cells
This work brings new information and a comprehensive picture of the pathobiology of Alzheimer’s disease and other diseases caused by tau proteins. It provides the groundwork for future intervention therapies specifically designed to improve clearing of Tau fibrils and to block their propagation throughout the brain.
Source
Fate and propagation of endogenously formed Tau aggregates in neuronal cells, EMBO Molecular Medicine, November 12, 2020.
Patricia Chastagner1, Frida Loria1, Jessica Y. Vargas1, Josh Tois1, Marc I. Diamond4, George Okafo2, Christel Brou1, Chiara Zurzolo1, 3.
1 Unité Trafic membranaire et pathogénèse, Institut Pasteur, Paris, France
2 GlaxoSmithKline, Gunnels Wood Road, Stevenage, R.-U., SG1 2NY
3 Correspondance : chiara.zurzolo@pasteur.fr
4 Center for Alzheimer’s and Neurodegenerative Diseases, Peter O’Donnell Jr. Brain Institute, University of Texas Southwestern Medical Center, Dallas, TX, États-Unis
This study is part of the priority scientific area Brain connectivity and neurodegenerative diseases of the Institut Pasteur's strategic plan for 2019-2023.





