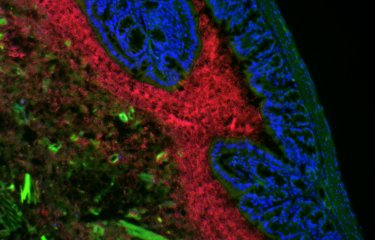
An international study has demonstrated that the microbiota and the intestinal cell death triggered by chemotherapy stimulate the efficacy of the immune response in patients with colorectal cancer. The results of the study, conducted in France by scientists from Gustave Roussy, Inserm, Université Paris-Saclay, the Institut Pasteur, IHU Méditerranée Infection and INRAE, were published in the journal Nature Medicine. The scientists offer new insights that shed light on the mechanisms governing positive or negative responses to cancer treatments.
With more than 43,000 new cases and 17,000 deaths each year, colorectal cancer is the second leading cause of cancer-related death in France. This type of cancer, especially right-sided tumors (in the proximal colon), which are more serious than left-sided tumors, have yet to benefit significantly from the two recent therapeutic breakthroughs in oncology – immunotherapy and precision medicine. Basic treatment with oxaliplatin remains the standard therapy for this digestive cancer. This chemotherapy triggers cell death (or apoptosis) in patients' intestinal cells, especially those in the ileum, the final section of the small intestine before the right colon. "The challenge was to understand the mechanisms behind the inefficacy of combination treatments with immunotherapy and oxaliplatin in patients with right-sided colorectal cancer," explains Professor Laurence Zitvogel, who led the study.
The scientists analyzed the conditions that trigger an immune response composed of unique cells known as T follicular helper (Tfh) cells, which are needed to immunize the host against cancer. In patients with colorectal cancer, they observed that treatment with oxaliplatin is more effective if two conditions are met:
- ileal crypt apoptosis,
- an ileal microbiota characterized by the presence of immunogenic bacteria (such as B. fragilis and E. ramosum).
These two conditions are associated with Tfh cells, which educate B lymphocytes and induce an immune response against cancer. "In the absence of these conditions, the death of ileal crypt cells triggered by oxaliplatin in the presence of an intestinal flora enriched in tolerogenic bacterial species is associated with a passive immune system and a poor prognosis for patients," explains Professor Zitvogel.
“In particular, we show that the activation of innate immunity by certain bacteria of the microbiota, via their surface structures, is essential to induce an immunogenic response against the tumor, while other members of the microbiota induce deleterious tolerance to anti-tumor treatment ”, explains Ivo Gomperts-Boneca, co-author of the study, and head of the Biology and Genetics of Bacterial Cell Wall Unit, at the Institut Pasteur (Paris).
In this study, the important role played by the microbiota and cell death in the efficacy of oxaliplatin treatment was demonstrated in mouse models whose microbiota had been suppressed by antibiotics or in which cell death was no longer possible.
The scientists also demonstrated in these models of vaccination with apoptotic intestinal cells (treated with oxaliplatin) that the efficacy of the vaccine can be regulated by the addition of immunogenic bacteria such as B. fragilis and E. ramosum, which induce an effective Tfh and B lymphocyte immune response.
In mouse models of colorectal cancer with no mutations, which would therefore not normally respond to anti-PD1 antibodies, they showed that adjusting the microbiota by adding immunogenic bacteria (B. fragilis and E. ramosum) restores the benefits of anti-PD1 immunotherapy.
Together, these results demonstrate the important role of the ileum (and not the colon) in the prognosis of colorectal cancer, and especially the role of the ileal microbiota and ileal cell death (not the colon microbiota and colon cell death) in the efficacy of patient response to cancer treatment in severe (proximal) colorectal cancer. The findings also pave the way for potential new strategies. "In future, patients with a genetic disorder such as Lynch syndrome that predisposes them to colorectal cancer may be able to be vaccinated with their own intestinal cells whose efficacy has been boosted with immunogenic bacteria. In the short term, as part of an upcoming clinical trial with 40 metastatic colorectal cancer patients treated with immunotherapy and chemotherapy, we are going to develop human ileum organoids, also known as enteroids, to further our knowledge of the microbial and immune parameters that dictate the efficacy of treatment," concludes Professor Zitvogel.
This study was supported by:
- The Ligue Contre le Cancer
- The French National Research Agency – RHU Torino Lumière (ANR-16-RHUS-0008)
- H2020 ONCOBIOME
- The French National Cancer Institute
- Cancéropôle Ile-de-France
- The Institut Universitaire de France
- LabEx Immuno-Oncology
- The Seerave Foundation
- SIRIC SOCRATE
- SIRIC Cancer Research and Personalized Medicine (CARPEM)
- FHU CARE
- Groupe Dassault
- The Paris Alliance of Cancer Research Institutes (PACRI)
- Elisabeth Badinter
- ITMO Cancer Aviesan (French National Alliance for Life Sciences and Health)
- The Centre National de la Recherche Scientifique (CNRS)
Source
Chemotherapy-induced Ileal Crypt Apoptosis and the Ileal Microbiome Shape Immunosurveillance and Prognosis of Proximal Colon Cancer, Nature Medicine, May, 25 2020 - doi : 10.1038/s41591-020-0882-8
Maria Paula Roberti1-3, Satoru Yonekura1,2,4, Connie P.M. Duong1,2, Marion Picard1,2,5, Gladys Ferrere1,2, Maryam Tidjani Alou1,2, Conrad Rauber1,2,4, Valerio Iebba1,2, Christian H.K. Lehmann6, Lukas Amon6, Diana Dudziak6, Lisa Derosa1-3, Bertrand Routy1,2,4, Caroline Flament1-3, Corentin Richard7,8, Romain Daillère1,2, Aurélie Fluckiger1,2, Isabelle Van Seuningen9, Mathias Chamaillard10, Audrey Vincent9, Stephanie Kourula11,12, Paule Opolon1, Pierre Ly1,2, Eugénie Pizzato1,2, Sonia Becharef1,2, Juliette Paillet4,13,14, Christophe Klein13, Florence Marliot15,16, Filippo Pietrantonio17,18, Stéphane Benoist19, Jean-Yves Scoazec20, Peggy Dartigues20, Antoine Hollebecque21, David Malka21, Franck Pagès15,16, Jérôme Galon15,16, Ivo Gomperts Boneca5,22, Patricia Lepage23, Bernard Ryffel24, Didier Raoult25, Alexander Eggermont1,2,21, Tom Vanden Berghe11,12, François Ghiringhelli7,8, Peter Vandenabeele11,12,26, Guido Kroemer13,14,27-29, and Laurence Zitvogel1-4,28
1 Gustave Roussy Cancer Campus (GRCC), 94800 Villejuif, France.
2 Institut National de la Santé Et de la Recherche Medicale (INSERM) U1015, Equipe Labellisée—Ligue Nationale contre le Cancer, 94800 Villejuif, France.
3 Center of Clinical Investigations in Biotherapies of Cancer (CICBT) 1428, 94800 Villejuif, France.
4 Univ. Paris-Sud, Université Paris-Saclay, Gustave Roussy, Villejuif, France.
5 Institut Pasteur Paris, Unit Biology and Genetics of the bacterial Cell Wall, 75015 Paris, France.
6 Department of Dermatology, Laboratory of Dendritic Cell Biology, Medical Immunology Campus Erlangen, University Hospital of Erlangen, Friedrich-Alexander-University (FAU) of Erlangen-Nürnberg, 91052 Erlangen, Germany.
7 Department of Medical Oncology, Center GF Leclerc, 21000 Dijon, France.
8 Plateform transfer in biological Oncology, 21000 Dijon, France.
9 Univ. Lille, Inserm, CHU Lille, UMR-S 1172 - JPARC - Jean-Pierre Aubert Research Center F-59000 Lille, France.
10 Univ. Lille, Inserm U1003 - PHYCEL - Physiologie Cellulaire, F-59000 Lille, France.
11 Molecular signaling and Cell Death Unit, VIB Inflammation Research Center, Ghent, Belgium.
12 Department of Biomedical Molecular Biology, Ghent University, 9000 Ghent, Belgium.
13 Equipe labellisée par la Ligue contre le cancer, Université de Paris, Sorbonne Université, INSERM U1138, Centre de Recherche des Cordeliers, Paris, France
14 Cell Biology and Metabolomics Platforms, Gustave Roussy Cancer Campus, Villejuif, 94805, France
15 Laboratory of integrative cancer immunology, INSERM U1138, Centre de Recherche des Cordeliers, 75006 Paris, France.
16 Service d’Immunologie Biologique, hôpital européen Georges Pompidou, 75005 Paris, France.
17 Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, 20113 Milan, Italy.
18 Department of Oncology and Hemato-oncology, university of Milan, 20122 Milan, Italy.
19 Service de Chirurgie Digestive et Oncologique, Hôpital Bicêtre, 94270 Le Kremlin-Bicêtre, France.
20 Departement de Biologie et Pathologie Médicales, Gustave Roussy Cancer Campus, 94800 Villejuif, France.
21 Departement de Médicine Oncologique, Gustave Roussy Cancer Campus, 94800 Villejuif, France.
22 INSERM, Equipe Avenir, Paris, France
23 Micalis Institute, INRA, AgroParisTech, Université Paris-Saclay, 78350 Jouy-en-Josas, France.
24 Molecular Immunology and Embryology, UMR 7355, CNRS, University of Orleans, Orléans, France.
25 Unité des Rickettsies, Faculté de Médecine, Université de la Méditerranée, Marseille, 13000 France.
26 Methusalem Program, Ghent University, 9000 Ghent, Belgium.
27 Pôle de Biologie, Hôpital Européen Georges Pompidou, Assistance Publique—Hôpitaux de Paris, Paris, France.
28 Suzhou Institute for Systems Medicine, Chinese Academy of Medical Sciences, Suzhou, China
29 Department of Women’s and Children’s Health, Karolinska University Hospital, 17176 Stockholm, Sweden
This study is part of the Cancer Initiative of the Institut Pasteur's strategic plan for 2019-2023.