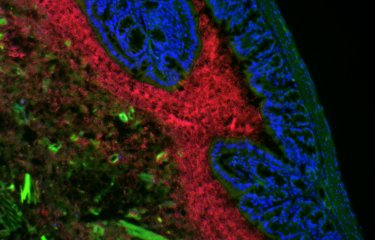
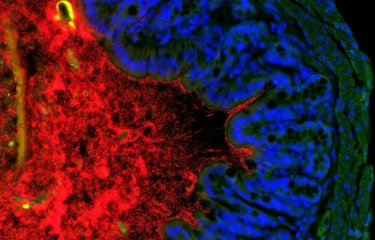
In achieving a state of equilibrium in the body, the billions of microbes that form assemblies known as microbiota at various sites including the gut, the skin and the oral cavity play an important role. The absence of microbiota results in increased exposure to some diseases. Scientists from the Bacteria-Cell Interactions Unit at the Institut Pasteur, in collaboration with INRA, Inserm, Georgia State University and Ghent University have discovered that Listeria bacteria produce a toxin – a bacteriocin – which affects their interactions with the gut microbiota and particularly targets the commensal bacteria Prevotella copri. This finding reveals a potential therapeutic strategy for some diseases, especially inflammatory diseases associated with an increase in Prevotella copri.
The gut microbiota plays an important role in protecting us from pathogens. Understanding the interactions between the host, the microbiota and pathogenic bacteria is vital for the identification of new therapeutic strategies based on manipulating the microbiota. But given the microbiota's vast complexity and the difficulty in culturing many species of commensal bacteria, some mechanisms have yet to be fully elucidated. Pathogenic bacteria have also developed sophisticated mechanisms to gain an advantage over gut bacteria and successfully infect the host.
Scientists in the Bacteria-Cell Interactions Unit at the Institut Pasteur set out to investigate the role and function of one of the genes of Listeria monocytogenes, an environmental opportunistic bacterium responsible for listeriosis. Listeriosis is a severe foodborne infection that causes septicemia and central nervous system infections. In France, listeriosis is relatively rare, with an incidence of 5 to 6 cases per million inhabitants, but it is fatal in 30 to 40% of cases occurring outside pregnancy (see our disease fact sheet). The gene, known as lmo2776, had not previously been studied, but it caught the scientists' interest because it is found in the majority of L. monocytogenes strains but is absent in L. innocua, a non-pathogenic species of the genus Listeria, suggesting that it may be implicated in L. monocytogenes' pathogenicity.
"Surprisingly and unexpectedly, in infections in animal models we observed that suppressing this gene increases L. monocytogenes' ability to colonize the gut and target organs such as the spleen and the liver, and also results in a reduction in the thickness of gut mucosa," explains Nathalie Rolhion, a scientist in the Institut Pasteur's Bacteria-Cell Interactions Unit and first author of the study. But suppressing the same gene had no impact on axenic animals, in other words animals with no gut microbiota. "This suggests that the effect of the gene lmo2776 depends on the microbiota," continues Nathalie Rolhion.
The importance of the microbiota in bacterial infections
Seeking to explain these results, the scientists demonstrated that the gene lmo2776 encodes a bacteriocin – a protein capable of inhibiting or killing other bacteria. This bacteriocin targets Prevotella bacteria in the microbiota in animals and humans. Prevotella is generally considered to be a commensal bacterium, in other words naturally present but with no impact on health. But several recent studies have demonstrated higher numbers of Prevotella copri in the gut microbiota of patients with rheumatoid polyarthritis, metabolic syndrome and low-level inflammation, suggesting that some strains of Prevotella could encourage and/or worsen some inflammatory conditions.
Pre-colonization of axenic animals by P. copri before infection by L. monocytogenes reproduced and mimicked the differences observed between infection with the wild bacterium and infection with the mutant strain in conventional mice (with a microbiota), whereas pre-colonization with all other commensal bacteria had no effect.
"This research demonstrates that P. copri can modulate gut infection and that pathogenic agents such as Listeria may selectively eliminate commensal bacteria from the microbiota to avoid excessive inflammation," concludes Pascale Cossart, Head of the Institut Pasteur's Bacteria-Cell Interactions Unit and last author of the study. "The corollary of this study is that lmo2776 may represent an effective therapeutic strategy in conditions associated withPrevotella copri, by reducing the quantity of P. copri in the gut without affecting the other bacteria in the microbiota."
This research led to the filing of an international patent published on August 15, 2019, number WO2019/155002, entitled "Anti-prevotella bacteriocin methods and compositions".
Source
A Listeria monocytogenes bacteriocin can target the commensal Prevotella copri and modulate intestinal infection, Cell Host & Microbes, 6 novembre 2019
Nathalie Rolhion1-3, Benoit Chassaing4,5, Marie-Anne Nahori1-3, Jana de Bodt6, Alexandra Moura7,8, Marc Lecuit7-9, Olivier Dussurget1-3,10, Marion Bérard11, Massimo Marzorati6, Hannah Fehlner-Peach12, Dan R. Littman12,13, Andrew T. Gewirtz5, Tom Van de Wiele6, Pascale Cossart1-3
1 Institut Pasteur, Unité des Interactions Bactéries-Cellules, 75015 Paris, France
2 Inserm, U604, 75015 Paris, France
3 INRA, Unité sous-contrat 2020, 75015 Paris, France
4 Neurosciences Institute, Georgia State University (GSU), 30303 Atlanta, Georgia, USA
5 Center for Inflammation, Immunity and Infection, Institute for Biomedical Sciences, GSU, 30303 Atlanta, Georgia, USA
6 Center of Microbial Ecology and Technology, Faculty of Bioscience Engineering, Ghent University, 9000 Ghent, Belgium s
7 Institut Pasteur, Unité Biologie des Infections, 75015 Paris, France
8 Inserm, U1117, 75015 Paris, France
9 Paris Descartes University, Sorbonne Paris Cité, Division of Infectious Diseases and Tropical Medicine, Necker-Enfants Malades University Hospital, Institut Imagine, 75743 Paris, France
10 Université de Paris, 75013 Paris, France
11 Animalerie Centrale, Department of Technology and Scientific Programmes, Institut Pasteur, 75015 Paris, France
12 The Kimmel Center for Biology and Medicine of the Skirball Institute, New York University School of Medicine, New York 10016, USA
13 Howard Hughes Medical Institute, New York 10016, USA