Expectations for a medical breakthrough in treating Alzheimer's and Parkinson's have never been higher. The two most common neurodegenerative disorders affect almost 900,000 and 200,000 people respectively in France – and given the aging population, the numbers affected by these age-related conditions will only continue to rise. By 2020, some 1.3 million individuals could suffer from Alzheimer's disease. So how much do we actually know about Alzheimer's and Parkinson's today?
Slow deterioration
Let's start with the more widespread of the two – Alzheimer's disease. The condition is rare in those under the age of 65 but it affects 15% of all 80-year-olds. The first symptom is short-term memory loss. This is followed by a decline in "executive function" – in other words, difficulties performing routine tasks such as using a telephone – and increasing disorientation in time and space – patients can lose their way on a familiar route or get confused about what time of day it is. They gradually lose their mental ability and independence, and begin to experience problems using language, writing and moving, behavioral symptoms, mood swings (anxiety, depression and irritability) and disturbed sleep. The disease progresses over several years, with the rate of progression varying from one person to the next.
What exactly goes on in the brains of those with Alzheimer's?
The neurons in several brain regions slowly begin to degenerate – especially in a structure known as the hippocampus, where short-term memories are stored – and this degeneration gradually spreads to the rest of the brain. Two culprits have been identified: the amyloid-beta peptide and the tau protein. The amyloid-beta peptide, which occurs naturally in the brain, accumulates until it forms "amyloid plaques" or "senile plaques". This build-up is toxic for nerve cells and is accompanied by changes to a protein involved in neuronal structure known as tau. The brain's neurons become disorganized, leading to neurofibrillary degeneration and cell death. This very slow neurodegenerative process begins affecting the brain years before the first symptoms appear.

Age, the main risk factor
The main risk factor of Alzheimer's disease is age, but genes also play a part. A combination of several genes may increase susceptibility to the disease. Some of these genes are involved in the metabolism of the amyloid peptide, some in inflammation, and some in interneuronal communication. There are also genes that provide individuals with protection against the disease.
Environmental factors also appear to play an important role. A sedentary lifestyle, frequent general anesthetics and uncontrolled cardiovascular risk factors (such as diabetes and high blood pressure) all seem to increase the likelihood of Alzheimer's. But a good education, a stimulating career and an active social life appear to slow down the emergence and severity of the first symptoms. These factors all boost our "cognitive reserve", which can be developed by stimulating the brain (see Interview, below) and seems to offer a functional replacement for lost neurons.
So how can Alzheimer's be treated? There are medications that slow the development of symptoms, together with other approaches, such as memory workshops and psychological treatment, which improve patients' quality of life. But there is currently no cure for Alzheimer's, which is why the research currently being carried out in laboratories is so important as it will pave the way for new therapeutic and diagnostic strategies.

Our brain is an echo chamber for our contacts with others – it is constantly nourished by what it gleans from our interactions with the outside world
What have we learned about the brain in the past few years?
Since the turn of the century, neuroscience has made several breakthroughs, one of which – maybe the most important – is that we have stopped seeing the brain as an isolated organ shut away in its skull. That was how Descartes viewed things – his "brain-centric" vision was that everything converged towards the brain, which was seen as a machine. But we now understand that the opposite is true: our brain is constantly interacting with both the external environment and our internal environment – hormones, chemical signals sent by bacteria in the gut microbiota, the blood and the factors it contains. These factors all influence brain function, because the brain is an open system. This is a major conceptual revolution. When devising new therapeutic strategies for neurodegenerative and other diseases, we now know that we can find resources within the body or in the outside environment.
Are there any therapeutic possibilities already based on this internal environment?
At the Institut Pasteur we are actively investigating the relationship between the microbiota and the brain, via a large-scale ANR [Agence Nationale de la Recherche, or French national agency for research]-funded program involving my team and two other research units. As soon as we are born, we are "invaded" by bacteria which colonize us and develop a symbiotic relationship with us – we provide the bacteria in our digestive tract with "board and lodgings" and in return they produce molecules for us that we can't create ourselves, such as vitamins. We are discovering that the microbiota is home to "psychobiotics", bacteria which secrete substances that influence our emotional reactions, our mood and our well-being. Basically, by taking different routes to access the brain, bacteria in the digestive tract take possession of the "control tower" that manages our actions, decisions and emotions. This has a huge impact, and this discovery opens up therapeutic prospects for psychiatric and neurodegenerative diseases that would have been unimaginable even just a few years ago, such as the development of treatments based on "friendly bacteria", probiotics, and strategies that treat inflammatory responses in some neurological and psychiatric conditions.
Is there anything we can do to keep our brain working properly?
Absolutely. Based on our discoveries about the adult brain's ability to repair itself and what we now know about behavior, we have come up with six golden rules [see opposite]. For example, we need to protect ourselves from information overload or "infobesity", which can lead to mental burn-out and harm our brain. Another crucial thing, which many people tend to ignore, is making an effort to interact with others, to develop our "social brain". People with genetic risk factors for Alzheimer's disease are less quick to develop symptoms if they have engaged in stimulating mental activity and have an active social life. In the light of what we are now discovering, our brain has a real appetite for developing contacts with those around us. "The other" can actually stimulate our own brain. Developing social links is vital.

Six golden rules to stimulate your brain
- Avoid routine and embrace change.
- Try to avoid information overload; favor useful information that improves understanding rather than pointless information.
- Keep away from anxiety medication and sleeping pills – long-term use can hinder the production of new neurons.
- Move and engage in physical exercise – your muscles produce substances that have a positive impact on the brain, via the bloodstream.
- Embrace otherness and foster social interaction, as some parts of your brain are stimulated when you open up to others.
- Take care over what you eat, since the composition of your gut flora can encourage or hinder the production of new neurons.
Parkinson's: loss of motor control
The second most common neurodegenerative disease in France is Parkinson's. The symptoms, which vary from one patient to the next, include difficulties starting to move, slowness of movement, increasingly small handwriting and problems writing, stiff limbs and involuntary shaking. As well as these motor control issues, Parkinson's can also give rise to often debilitating symptoms such as extreme tiredness, loss of smell, anxiety, and problems swallowing and speaking.
As with Alzheimer's, the disease actually begins many years before the first symptoms become visible. Parkinson's disease destroys a specific neuronal population – dopaminergic neurons in the substantia nigra in the brain. Dopamine is a neurotransmitter (a molecule that enables neurons to communicate with each other) involved in regulating movement, which explains the loss of motor function in Parkinson's patients. The brain initially compensates for the loss of dopamine, maintaining normal function. The first symptoms only begin to appear when 50 to 70% of dopaminergic neurons have been destroyed.
This degeneration is caused by a toxic build-up of proteins, known as "Lewy bodies", in the substantia nigra and other brain regions. These pathogenic aggregates are formed by the protein alpha-synuclein (α-synuclein), which in Parkinson's patients adopts an abnormal conformation that encourages aggregation.
A new therapeutic target and possibilities for cell therapy
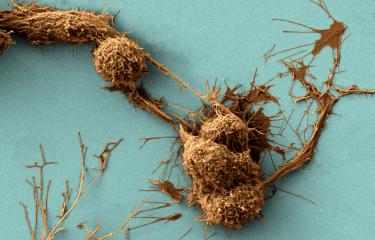
Scientists at the Institut Pasteur recently made a major discovery concerning a protein involved in Parkinson's disease. In Parkinson's patients, this protein, α-synuclein, is "misfolded" and forms clumps in the brain, potentially leading to neuronal death. But how does α-synuclein spread? The Membrane Traffic and Pathogenesis Unit, directed by Chiara Zurzolo, used fluorescence microscopy to show that, in culture, pathogenic α-synuclein fibrils travel between neurons through "nanotubes", recently discovered structures involved in intercellular communication. "These nanotubes therefore represent a new therapeutic target to stop the progression of Parkinson's disease and other neurodegenerative conditions such as Alzheimer's, Huntington's chorea and prion diseases, which involve other "misfolded" proteins that also spread via nanotubes. We are currently studying these different proteins in my laboratory," explains Chiara Zurzolo.
Her team, together with the Perception and Memory Unit directed by Pierre-Marie Lledo and the Hellenic Pasteur Institute in Athens, has also launched a research project on skin cells in both healthy subjects and individuals affected by a subset of familial Parkinson's, who have a mutant α-synuclein gene. Using a method that has now become widespread, these skin cells are turned into stem cells, which are then differentiated into neuronal precursors (neuroblasts). In experimental models, these neuroblasts are injected into a region of the brain where new neurons naturally mature (the olfactory bulb), which is rich in dopaminergic neurons – the same neurons that are destroyed in Parkinson's disease. "We want to see whether these human neuroblasts can differentiate into dopaminergic neurons, and our preliminary results are encouraging," explains Françoise Lazarini, a scientist in the Perception and Memory Unit. "We are also going to analyze the effect of neurons from patients containing the mutant gene, and see if they can trigger Parkinson's disease. We are hoping to pave the way for new cell therapy possibilities and improve our understanding of the mechanism underpinning the disease, especially the role of α-synuclein."

An occupational disease in the agricultural sector
As with Alzheimer's disease, it is hard to pinpoint the exact causes of neurodegeneration in Parkinson's, and age is again the main risk factor. But other genetic and environmental factors also seem to play a role. The link with pesticide exposure, for example, has been confirmed – farmers exposed to organochlorine pesticides are more likely to develop Parkinson's, which is recognized in France as an occupational disease in the agricultural sector. Smoking and coffee seem to offer protection against the condition, possibly because nicotine and caffeine stimulate dopaminergic neurons.
Current treatments for Parkinson's include dopamine precursors (levodopa or L-dopa) and molecules that mimic the effect of dopamine, or enzyme inhibitors which block the action of enzymes that break down dopamine. While these drugs effectively treat the motor symptoms associated with the condition, they do not prevent the progression of neurodegeneration, and complications occur after five to ten years of treatment. As well as medication, physiotherapy is offered to help maintain healthy muscles and joints, facilitate walking and improve balance.

© William Beaucardet
Diagnosing the early stages of the disease
There is currently no way of diagnosing the early stages of Alzheimer's – despite the fact that, on average, 20 years go by between the onset of the disease and dementia. In the Institut Pasteur's Antibody Engineering Platform, directed by Pierre Lafaye, scientists have developed a technique for detecting the first lesions caused by Alzheimer's – using llama antibodies! "We use fragments of llama antibodies, known as "nanobodies", which are able to cross the blood-brain barrier that surrounds and protects the brain," explains Pierre Lafaye. "Specific nanobodies targeting the two proteins associated with the disease, the tau protein and the amyloid-beta peptide, are paired with a contrast agent so that they can be revealed using MRI once they have bound to the target proteins." These two "nanoprobes" have proved effective in detecting very early lesions in experimental models. Developing this method may offer new hopes of treating patients earlier and more effectively.

A wide range of therapeutic possibilities
For patients that demonstrate resistance to medication and are physically strong enough to cope with the surgery required, deep brain stimulation can be beneficial. This technique involves implanting electrodes into a specific brain region, together with a device under the skin that constantly emits high-frequency electrical impulses.
Scientists have also conducted cell therapy trials, transplanting functional neurons into Parkinson's patients to replace dead or degenerating neurons. These trials have led to mixed results. This type of "restorative" therapy is currently the focus of extensive research, especially using stem cells, and represents a promising avenue for all neurodegenerative diseases. Scientists are actively looking for new approaches to tackle these devastating conditions, exploring all possible avenues – from repairing the brain to searching for "rejuvenating molecules" (see below) and new therapeutic targets (see below) – with the aim of developing effective treatments for the future.
The promise of a "rejuvenating molecule"
We already know that there are "rejuvenating factors" in the blood: experiments have shown that treating old organisms with blood from young organisms has rejuvenating effects. Lida Katsimpardi studied the effect of "young blood" on the aging brain while she was at Harvard University. "In experimental models, administering young blood increases the production of new neurons, leading to vascular remodeling in the cortex and other areas. This improved vascularization increases blood flow, which could stimulate neuron activity," explains the scientist, who also showed in 2014 that a blood factor known as GDF11 was capable of inducing the same effects on its own. Lida Katsimpardi, now based in the Institut Pasteur's Perception and Memory Unit, is actively researching other rejuvenating molecules. "I believe that there is an "elixir of youth" waiting to be discovered. This research offers promising new therapeutic possibilities. We will soon be testing the effect of GDF11 in an Alzheimer's disease model."

The nicotinic receptor, a target for Alzheimer's disease

The nicotinic acetylcholine receptor, or nAChR, was identified in 1970 by Prof. Jean-Pierre Changeux at the Institut Pasteur, where it is still a key focus of research. As well as binding to nicotine, the nAChR also naturally responds to acetylcholine, a neurotransmitter involved in motor control, memory and learning. In Alzheimer's disease, acetylcholine levels are reduced, since the neurons that produce it are the first to be affected by the degenerative effects of the condition. The only treatments currently administered for Alzheimer's are molecules that prevent acetylcholine degradation. "Our hope is that in the future we will be able to act earlier to tackle the disease and even to offer curative treatments," explains Uwe Maskos, Head of the Integrative Neurobiology of Cholinergic Systems Unit. "We have successfully modeled the events that lead to the early stage of the disease, especially how the amyloid-beta peptide changes the brain's activity when it binds to the receptor." Scientists are currently looking for "nicotine-like" components that can specifically block this dangerous binding activity and can be administered safely.

Brain "plasticity" and repair

Our brain is extraordinarily dynamic. This 1.5kg organ, which uses up 20% of the body's energy, is constantly changing, regenerating and even repairing itself. Since the turn of the 21st century, we have known that two brain regions in adults (the hippocampus and the subventricular zone/olfactory bulb) produce new nerve cells. But how do these new neurons develop, and how are they integrated into existing neuronal networks? Since 2010, an original technique has been used to selectively stimulate new neurons that have been made sensitive to photons and can therefore be activated by light flashes. "Using optogenetics, we can understand the role of a specific neuron and identify the other neurons it communicates with, its purpose, etc.," explains Gabriel Lepousez, from the Institut Pasteur's Perception and Memory Unit. "We have demonstrated that a new neuron can only be properly integrated into a circuit by establishing multiple connections with other neurons; otherwise it self-destroys. Its integration is also influenced locally by inflammation or the immune system, and by factors outside the brain such as the microbiota." So can we look forward to cell therapy techniques in which new neurons are implanted to repair brain regions which have been damaged by a neurodegenerative disease or a stroke? "Research into brain plasticity is the key to these future therapies," emphasizes the scientist. "But we mustn't get ahead of ourselves. The more we study the brain, which functions with millisecond precision, the more we are realizing that we previously underestimated its complexity. We have never before had access to such powerful tools, and physicists and mathematicians are helping us to model our data so that we can analyze them more effectively. But repairing damaged circuits in the brain requires incredibly high-precision medicine, and we need to proceed with the utmost caution when treating such a complex organ that is the very hub of our consciousness and personality."