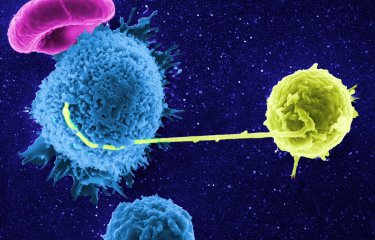
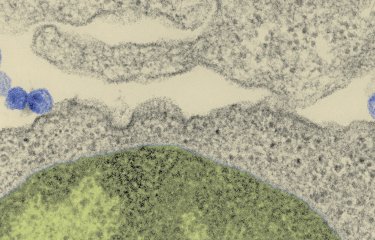
Rare individuals infected by the AIDS virus control the infection and do not develop the disease, in spite of more than ten years of being HIV positive and in the absence of treatment. A team from the Institut Pasteur and a team from Inserm U802, in collaboration with clinicians from Bicêtre Hospital, have just explained how these individuals succeed in controlling HIV. Their study, conducted under the aegis of the ANRS and published in 'PNAS', could have important implications for the immunotherapy of AIDS and vaccine research.
Press release
Paris, april 10, 2007
In 2005, Prof. Jean-François Delfraissy's team from the Internal Medicine department at Bicêtre University Hospital identified exceptional patients infected by HIV-1 called "HIV Controllers" (HIC).In these very rare individuals (less than 1% of those with HIV), the multiplication of the virus is not (or only rarely) detected in their blood, in the absence of treatment, during more than 10 years of being HIV positive. A study (ANRS EP36), coordinated by Dr. Olivier Lambotte, was set up by the ANRS in order to study the reasons for such control.
Indeed, the natural ability of the HICs to control the infection could be imitated by therapies or by an adapted vaccine. However, the mechanisms of this control were yet to be understood.
That is what has just now been brought to light by a study conducted by Asier Sàez-Ciròn* and Gianfranco Pancino, in the Regulation of Retroviral Infections Unit at the Institut Pasteur (unit directed by Françoise Barré-Sinoussi), and Alain Venet from Inserm Unit 802 at the Université Paris XI Medical School in Le Kremlin-Bicêtre, within the framework of the ANRS EP36 study.
The study concerned 11 HIC patients, some of whom were diagnosed starting in 1983. The researchers first demonstrated that their CD4 T cells, the target cells of HIV, were sensitive to the virus, as in the general population, and that there was thus no intrinsic resistance to infection. Further, they showed that these individuals were truly infected by replicating viruses.
Their research then centred around the response of other T cells, CD8 T cells: until now, it was known that this population of cells from the immune system was preserved and functional in HIC patients, unlike patients who develop the disease. The main role of these cells is cytotoxic: special CD8 cells called CTL (cytotoxic T lymphocytes) are true killer cells able to recognise infected cells very specifically and kill them. First, the researchers confirmed that the HIC individuals have a particular gene pool, one likely to encourage immune response, in agreement with previous studies.
The new information provided by this study is that the CD8 T cells of "HIV controllers", brought into contact with their own CD4 T cells, are capable, without any previous stimulation, of totally inhibiting their infection in vitro. There is no remaining trace of viral production in the cultures of infected CD4 T cells, and the study demonstrates that this is due to a powerful and rapid destruction of infected cells.
Even more importantly, the researchers succeeded in characterising the profile of these specific, highly cytotoxic CD8 T cells of HIV. In comparing the CD8 T cells of HIC patients with those of non-controller subjects, they showed that two "activation markers" from these lymphocytes-molecules on their surface-were expressed in very different quantities in the two patient populations.
The possibility of using these markers to spot the CD8 T cells specific to HIV that are particularly effective in controlling the virus could serve to better direct vaccine research and immunotherapy-using these methods in an attempt to stimulate the immune system, to obtain CD8 T cells of the same profile as those of HIC patients. "These markers, as well as the control test for the infection in vitro developed in the study, could now be used to evaluate the effectiveness cytotoxic T cells response in vaccine trials", the authors suggest.
Their investigations on these "HIV controllers" are continuing. In the future, their studies should be facilitated by the creation of a national information centre to record HIC patients, under the aegis of the ANRS.
* Asier Sàez-Ciròn is the recipient of a grant from Sidaction.
Sources
"HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype" : PNAS, april 2007.
Asier Sàez-Ciriòn (1), Christine Lacabaratz (2), Olivier Lambotte (2,3), Pierre Versmisse (1), Alejandra Urrutia (2), Faroudy Boufassa (4), Françoise Barré-Sinoussi (1), Jean-François Delfraissy (2,3), Martine Sinet (2), Gianfranco Pancino (1), Alain Venet (2) for the ANRS EP36 HIV Controller Study Group.
1. Regulation of Retroviral Infections Unit, Institut Pasteur, Paris
2. Unit 802, Inserm, Faculty of Medicine, Université Paris XI, Le Kremlin-Bicêtre
3. Internal Medicine and Infectious Diseases Department, Bicêtre Hospital, Le Kremlin-Bicêtre
4. U 569, Inserm, Bicêtre Hospital, Le Kremlin-Bicêtre
Contact persons
ANRS Press Office
Marie-Christine Simon
+ 33 (0)1 53 94 60 30
marie-christine.simon@anrs.fr
Institut Pasteur Press Department
Nadine Peyrolo or Corinne Jamma
+ 33 (0)1 40 61 33 41
cjamma@pasteur.fr
Inserm - Press Office
Séverine Ciancia
+33 (0)1 44 23 60 86
presse@tolbiac.inserm.fr