Type 1 diabetes, multiple sclerosis, rheumatoid arthritis, ankylosing spondylitis, psoriasis, systemic lupus erythematosus, vitiligo, Crohn's disease and Guillain-Barré syndrome – these seemingly different diseases all have one thing in common: they occur when a deregulation of the immune system causes it to "attack" the organism that it is supposed to be protecting. These "autoimmune" diseases, which currently affect around 5 million people in France, are the third most common category of disease in industrialized countries in terms of morbidity and mortality, after cancer and heart disease. Although there are treatments to slow down the progression of autoimmune diseases, there is currently no cure.
Our "self" becomes the enemy
The role of the immune system, an inner army composed of several types of white blood cells, is to defend the body from external aggression such as bacteria or viruses. It generally tolerates its own constituents, known as the "self". But when this tolerance breaks down, the immune system can itself become a source of disease. Some white blood cells (known as "self-reactive lymphocytes") specifically attack tissues or organs. Antibodies, normally produced by immune cells to neutralize the "enemy" by binding to some of its molecules (antigens), can also emerge and target areas of our body.
In type 1 diabetes (see inset below), for example, autoantibodies target the insulin-secreting cells in the pancreas. In rheumatoid arthritis, the membrane that lines the joints is targeted, and the ensuing inflammation can then spread to the cartilage and bones, and sometimes even to the tendons and ligaments. In systemic lupus erythematosus, autoantibodies attack the molecules on many cells in the body, resulting in lesions in several organs (including the skin, kidneys and heart).

Type 1 diabetes: a key gene under study
Type 1 diabetes, sometimes referred to as juvenile diabetes (onset is generally in childhood), is responsible for 10% of diabetes cases worldwide. This autoimmune disease, caused by the destruction of insulin-secreting cells in the pancreas, requires daily injections of the vital hormone. In the Immunology of Diabetes team at the Institut Cochin, Ute Rogner is studying the action of a gene involved in this disease that she first identified at the Institut Pasteur in 2006. This gene seems to be the source of the disease. Its role is to produce a protein (Arntl2) that regulates an immune molecule responsible for the proliferation of diabetogenic cells (interleukin 21). "The aim of our research is to develop a precise understanding of the entire chain of molecular and cellular events triggered by Arntl2," explains Ute Rogner. "This research should reveal new therapeutic targets. We are also hoping to find markers that will enable earlier detection of type 1 diabetes in families at risk, and Arntl2 may be one such marker." The gene is particularly interesting as it also seems to be linked to type 2 diabetes.

Chronic inflammatory diseases
Some conditions that do not involve autoantibodies are referred to instead as "autoinflammatory" syndromes. Immune cells in the body's first-line defense mechanism (including neutrophils, macrophages, monocytes and natural killer cells) trigger a chronic inflammation on their own, leading to the destruction of various tissues: the skin in psoriasis (which affects 3 to 5% of the European population), some joints in rheumatoid arthritis (see inset), the digestive tract in Crohn's disease, and the central nervous system – the brain, spinal cord and optic nerve – in multiple sclerosis (see inset below). Whether autoimmune in the strict sense of the term or autoinflammatory, all these conditions are the result of immune system dysfunction and develop into chronic inflammatory diseases.
Neutrophils © Institut Pasteur
Improving the treatment of ankylosing spondylitis
Ankylosing spondylitis is a debilitating, painful condition that is as widespread as rheumatoid arthritis. It affects some 300,000 people in France and nearly one and a half million across Europe. This chronic autoinflammatory disease, which occurs most often in young men (aged between 20 and 40), affects the sacroiliac joint in the spine and sometimes also joints in the arms and legs. Inflammation is accompanied by the formation of new bones which can cause sections of the spine to "fuse" together. Spinal flexibility becomes severely limited, leading to an increasingly stooped posture. This disease, which has a strong genetic component, is being investigated at the Institut Pasteur in the Immunoregulation Unit directed by Lars Rogge, who in 2016 set up a joint research unit on ankylosing spondylitis with the Rheumatology Department at Cochin Hospital.
"We are particularly interested in the molecules used to treat the disease, which block an inflammatory cytokine known as TNF, and we have recently elucidated their mechanism of action," explains the scientist. "This could open up new approaches to the disease and also enable us to identify signs in patients that predict whether they will respond effectively to anti-TNF drugs, which have no effect in a third of patients. By analyzing 70 patients, we have identified candidate molecules that we hope to use to define biomarkers for the therapeutic response. We are now launching a study on 200 patients to explore these avenues and improve our understanding of the mechanism of action of another molecule that has recently been shown to be of interest for treating ankylosing spondylitis, namely anti-IL17. The cohort of healthy volunteers in the Milieu Intérieur project (see inset below) will also enable us to compare the immune responses of healthy individuals and ankylosing spondylitis patients."
The goal? To develop tools that will help doctors choose the most appropriate treatment for their patients.

Lars Rogge, head of the Immunoregulation Unit at the Institut Pasteur.
The cohort of healthy volunteers in the Milieu Intérieur project will also enable us to compare the immune responses of healthy individuals and ankylosing spondylitis patients.
Multiple risk factors
Why does this deregulation happen? We still know very little about the causes (see Interview below), but several factors are associated with the onset of autoimmune diseases, including sex hormones, since women are affected more often than men – 80% of cases of autoimmune thyroiditis, for example, occur in women (and 3 to 5% of women are affected by the disease).

Genetic makeup is also important, with many of these conditions running in families. The frequency of type 1 diabetes, for example, rises from 0.4% among the general population to 5% in individuals with a diabetic family member. Dozens or even hundreds of genes, often involved in immunity, have been associated with each autoimmune disease. Some play a part in the biological identity of the individual, coding for self-recognition proteins located at the cell surface, such as the gene HLA-B27, found in more than 80% of ankylosing spondylitis patients but just 7% of healthy people.
The environment – exposure to germs, chemicals, UV rays, tobacco, etc. – also plays a major role. Some claim that the increasing prevalence of autoimmune diseases in industrialized countries is caused by improved hygiene and the use of antibiotics. It does appear that a lack of exposure to infection may be a risk factor.

In general, a single factor is not enough to trigger an autoimmune disease. But each factor has a small effect, and the effects of all those factors together give rise to the disease.
Where does the immune deregulation that causes autoimmune diseases come from?
There are several theories. One theory that has been around for a long time claims that we attack ourselves because some elements of the self resemble microbes: when we are infected by a virus, the antibodies produced to tackle an antigen of this virus "see" a component in our body that is very similar and target it instead. This is the notion of "antigen mimicry". Very specific examples have been documented, but it remains to be proven in diseases such as multiple sclerosis and rheumatoid arthritis. Another more modern theory is that those with autoimmune conditions simply have a stronger tendency to react – their immune responses are triggered at the slightest provocation. Our immune system can naturally react against the self, but this reaction is generally regulated and moderated. In some people, however, either this natural check is absent, or the reaction is too powerful. The mechanism of action of some genes associated with autoimmune diseases appears to back up this theory. A third hypothesis is that the overactivation of the immune system is caused by an infection. After coming into contact with a microbe, the immune system is activated to such an extent that it reacts more readily against the self.
Why is it so hard to identify the mechanisms involved?
Identifying the causes of cystic fibrosis is relatively easy: the disorder is caused by a single mutant gene. But chronic inflammatory diseases develop over decades and are influenced by a huge number of risk factors. Research on the genomes of thousands of people has shown that a considerable number of genes are involved – hundreds for Crohn's disease alone. And then there are environmental and lifestyle factors, eating habits, etc. In general, a single factor is not enough to trigger an autoimmune disease. But each factor has a small effect, and the effects of all those factors together give rise to the disease. That's why studying these diseases is so complicated.
You are an immunologist and the focus of your research is the gut microbiota. Could you tell us a little more?
We are trying to understand the role played by the gut microbiota in regulating the immune system. We know that there is a sort of dialog between gut bacteria and immune cells. We are trying to identify the rules governing this interaction, and how it can be deregulated, by working on healthy systems and on models of colitis and arthritis. It has been shown that autoimmune diseases lead to a reduction in the diversity of the microbiota. So there could be a loss of some bacterial strains that protect us from these diseases. For example, the bacterium Faecalibacterium prausnitzii can be found in healthy individuals but is missing in some patients with chronic inflammatory bowel disorders. One option to explore could be re-implanting these bacteria in patients.
So could research on the microbiota result in new treatments?
That's the idea. But although we are now able to study the bacteria in the microbiota one by one, in reality they are in constant communication with their billions of neighbors in the gut. Bacterium A needs bacterium B, which needs bacterium C, and so on. We are trying to unravel an extremely complex ecosystem. Another problem is our ability to culture beneficial bacteria, and we don't necessarily have all the conditions we need to manipulate them yet. But as we continue to investigate why and how a given bacterium protects us, we will undoubtedly identify substances that it produces which are involved in regulating the immune system. We can then focus our attention on synthesizing these products.
"We know that there is a sort of dialog between gut bacteria and immune cells. We are trying to identify the rules governing this interaction."
Taming the immune system
Apart from type 1 diabetes, which is treated by replacing missing insulin, most autoimmune diseases are treated by methods to control and reduce the immune and inflammatory responses.
Corticosteroids, known for their anti-inflammatory properties, are the standard therapy but can have harmful long-term effects. Immunosuppressive drugs such as cyclosporine, also used to prevent organ rejection in transplant patients, may be administered. But by suppressing the autoimmune reaction, they also reduce the body's ability to defend against microorganisms (bacteria, viruses, etc.) or to eliminate cancer cells. This risk of developing infection or cancer means that patients need to be carefully monitored.

Photo from LabEx Milieu Intérieur. Credit: Institut Pasteur
What is a "healthy" immune system? The "Milieu Intérieur" project
The immense complexity of immune responses in individuals and populations previously prevented scientists from identifying the parameters – both genetic and environmental – that create a healthy immune system and are responsible for its natural variability. The "Milieu Intérieur" consortium, coordinated at the Institut Pasteur* and composed of 30 teams from a variety of research institutions and hospitals, is aiming to characterize the immune system of healthy individuals. Since 2012, these scientists, who specialize in several disciplines (immunology, genomics, molecular biology and bioinformatics), have been working on a large-scale study of 1,000 healthy volunteers. "This research will pave the way for personalized medicine, in other words tailoring a therapeutic strategy to a given person at a given time." The Milieu Intérieur cohort is also proving useful for several research teams working on autoimmune and autoinflammatory diseases as a means of comparing the immune characteristics of patients and healthy individuals.
The Milieu Intérieur Consortium is a "Laboratory of Excellence" or LabEx under the French government's Investing in the Future program.
* by Lluis Quintana-Murci, Director, and Darragh Duffy, Coordinator.

The rise in biopharmaceuticals
A third category of treatments for autoimmune diseases is biopharmaceuticals. This approach involves either administering or inhibiting "cytokines", the messenger molecules produced by immune cells. The mainstay of biopharmaceutical treatments is "anti-TNF-alpha" drugs, which since 1997 have sparked somewhat of a revolution in rheumatology, with spectacular results on rheumatoid arthritis. As their name suggests, these drugs block TNF-alpha, a cytokine which activates inflammation. They are currently also prescribed for Crohn's disease and psoriasis. Cytokines involved in the regulation of the immune system are also used, such as interferon beta in multiple sclerosis (see inset below). Unlike traditional immunosuppressors, which act on the immune system as a whole, these biopharmaceuticals target specific pathways, reducing a given population of immune cells or stopping them from migrating to the organs affected by the disease. They are the focus of a huge number of trials worldwide. The rapid growth in these approaches is a result of our increasingly detailed understanding of the events that take place in each autoimmune disease and the precise role played by each cytokine.
Multiple sclerosis: hopes raised by biomarkers and radically new treatments
Multiple sclerosis (MS) is an autoimmune, neurodegenerative disease that affects 2.5 million people worldwide, 80,000 in France, and three times more women than men. It occurs as a result of the progressive destruction of the protective coating surrounding nerve cells in the brain. Symptoms of multiple sclerosis generally begin around the age of 30, with bursts of inflammation, sometimes several months apart, after which the patient recovers – but less and less over time. After this first stage, which lasts between 5 and 20 years, a second "progressive" stage begins, leading to a worsening of sensory and muscular problems and often difficulties walking. Risk factors include a genetic predisposition, environmental factors such as a lack of sunlight and latitude (MS is much more common at the two poles), a history of glandular fever or obesity in adolescence, and nicotine dependence.
Predicting the response to interferon treatment
Treatment involves administering cortisone and immunomodulators, especially interferon beta, which reduces the frequency of relapses by around 30% and the frequency of new lesions by about 60%. "This interferon has been used to treat multiple sclerosis since 1993, but we know relatively little about its mechanism of action," says Frédérique Michel from the Cytokine Signaling Unit at the Institut Pasteur, directed by Sandra Pellegrini. "It is a fascinating molecule as it has three very different properties: it has an antiviral effect – it is one of the first immunity molecules to be secreted when the body is attacked by a virus –; it is used to treat some forms of cancer because it inhibits cell proliferation; and finally it is involved in regulating the immune system – and it is this third property which interests us. We are currently working with neurologists from Pitié-Salpêtrière Hospital in Paris to carry out a study on the immune response of patients treated with interferon beta. We are using sophisticated new technological approaches to analyze hundreds of genes and proteins and the cell populations involved in controlling the immune response. We are hoping to discover biomarkers for the therapeutic response, since around 30% of patients currently fail to respond to this treatment." The scientists are investigating various avenues of research in around 50 patients, raising hopes for future improvements to multiple sclerosis treatment.

Frédérique Michel, Cytokine Signaling Unit a the Institut Pasteur.
It is a fascinating molecule as it has three very different properties.
Taking back control of immunity genes
In another laboratory, Christian Muchardt, Head of the Epigenetic Regulation Unit, is exploring an entirely different approach. "Viral proteins have been found in the white blood cells of individuals with multiple sclerosis," he explains. "They are not there because of a viral attack; instead they come from the activation of virus genetic sequences that have been integrated into our DNA over the course of evolution – we might refer to them as viral remnants. In healthy individuals, they are kept under control." Christian Muchardt's team set out to try to understand why this state of control is disrupted in MS patients. The scientists identified an epigenetic* mechanism responsible for this deregulation, and demonstrated that the mechanism also deregulates immunity genes. "We are now trying to correct the defective mechanism with molecules that will serve as good candidates for future multiple sclerosis drugs."
*Involving proteins that are associated with DNA to reduce or increase gene expression.

Christian Muchardt, head of the Epigenetic Regulation Unit at the Institut Pasteur.
We are now trying to correct the defective mechanism with molecules that will serve as good candidates for future multiple sclerosis drugs.
The microbiota and cell therapy
Finally, autoimmune diseases are also set to benefit from current research in two areas: the microbiota, since our gut bacteria appear to be involved in immune system regulation (see Interview), and stem cells – damaged organs may be restored by injecting cells that are able to differentiate and function locally, for example rebuilding cartilage to "repair" joints in arthritic conditions.
A number of different research fields are therefore raising hopes that more powerful, targeted treatments will be developed to treat these complex diseases. The aim is to repair the errors made by our immune defenses, which can sometimes be wildly off target...
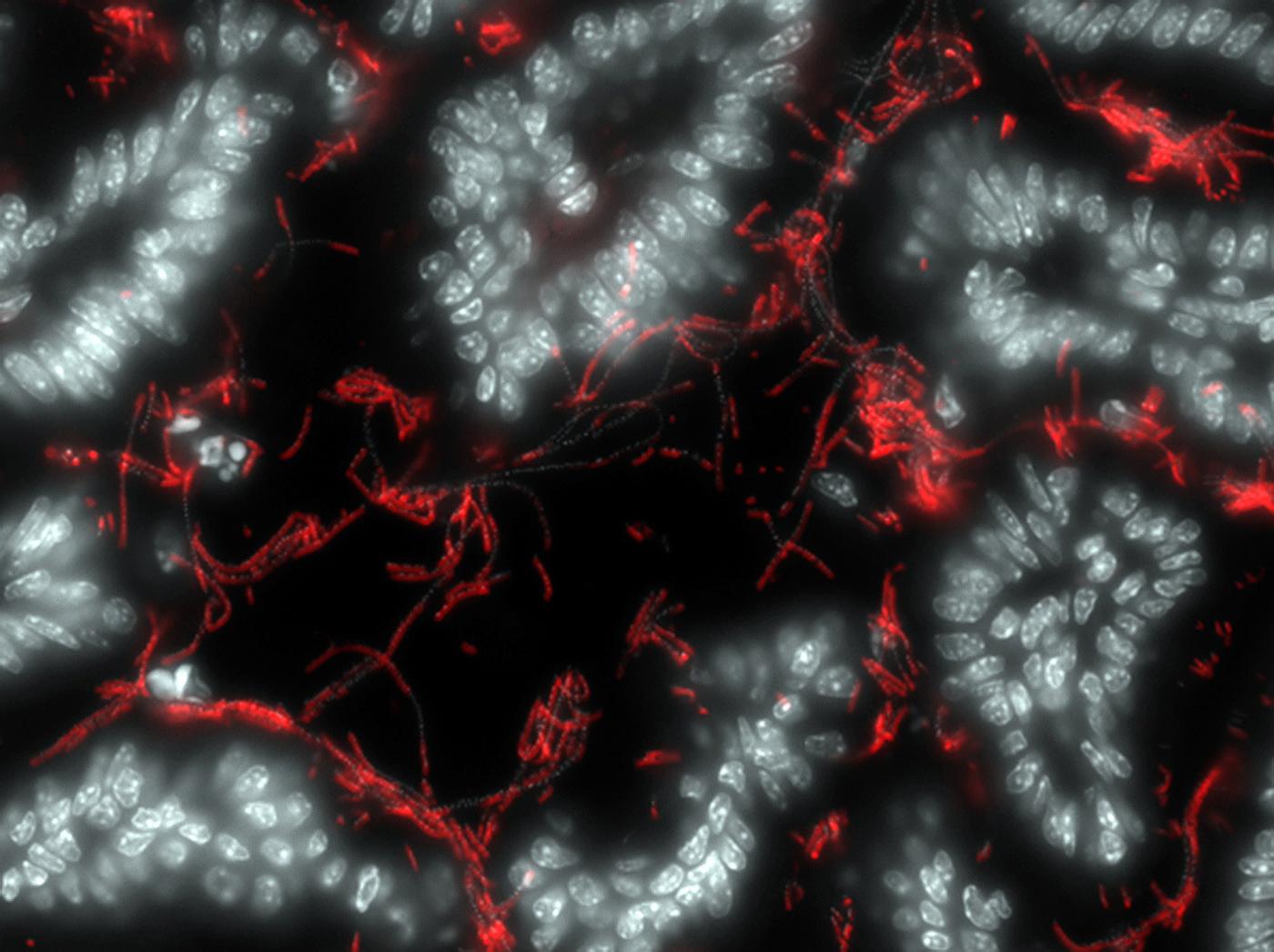
Symbiotic bacteria (red) attached to the intestine (in white). © Institut Pasteur