Ebola, plague, cholera, SARS… Emerging pathogens, an unpredictable threat

One hundred years after the deadliest influenza pandemic in history – the Spanish Flu of 1918 –, emerging and re-emerging infectious diseases continue to represent a major threat to public health worldwide. As the international Emerging Infections and Pandemic Risk conference was held at the Institut Pasteur on June 21 and 22, the recent Ebola outbreak in the Democratic Republic of the Congo came to an end. The Institut Pasteur recently sent a young researcher to the country to investigate the outbreak and help tackle any future occurrences of Ebola.
Let's start with a brief history. In the 14th century, the Black Death spread from the banks of the Mediterranean to Scandinavia. It is believed that the pandemic wiped out 30 to 50% of the entire European population over a five-year period (1347-1352), claiming approximately 25 million lives. It took two centuries for the European population to return to its previous levels. The Black Death was a form of plague caused by a bacillus (subsequently discovered by Alexandre Yersin) transmitted by fleas living on rats. When humans were infected, they developed buboes (similar to large blisters), resulting in septicemia and sometimes death. The Black Death has long been held up as an important historical example – but in August 2017 history almost repeated itself.
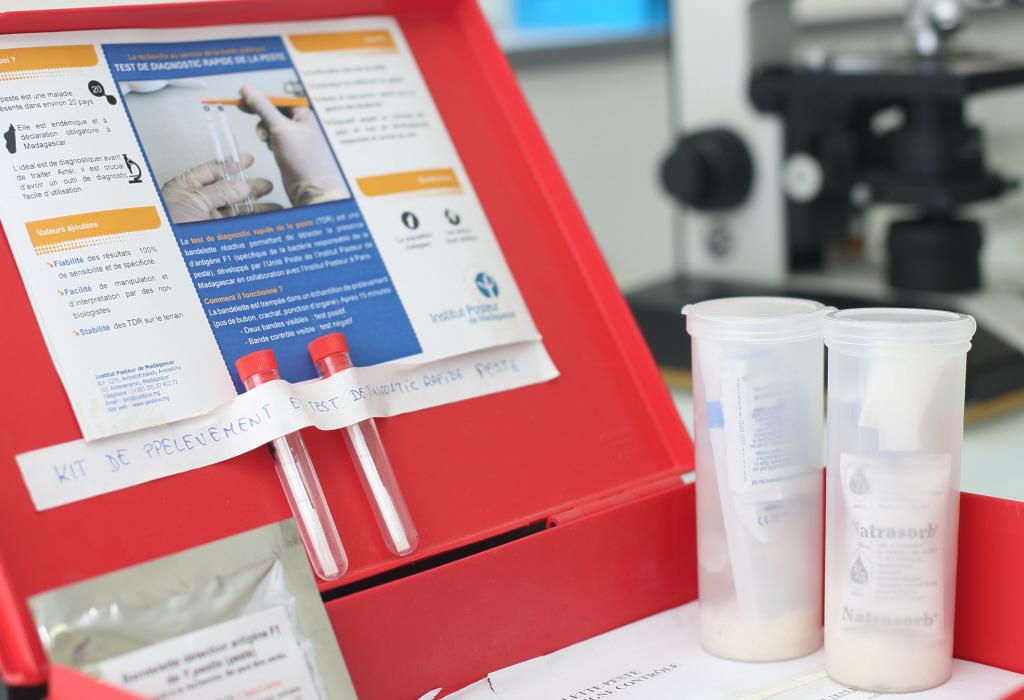
From medieval plague to current risk
Madagascar is regularly affected by sporadic cases of plague, but last year a patient suffering from a highly contagious pneumonic form – which can be spread just by coughing – took a taxi from the Madagascan highlands to the capital. By the time he arrived in Antananarivo, he had come into contact with 31 other people in the taxi, of whom 10 became infected and 4 died. This was the start of an outbreak of pneumonic plague in the Madagascan capital of Antananarivo and more generally over the whole island. "We were very concerned as epidemiologists," explains Prof. Arnaud Fontanet, Head of the Epidemiology of Emerging Diseases Unit at the Institut Pasteur. "We were witnessing an outbreak of a disease that can spread through person-to-person transmission in a capital city with 2 million inhabitants, in a country known to have a fragile healthcare system. With a team of nine people from the Institut Pasteur in Paris, we set off to support our colleagues at the Institut Pasteur in Madagascar." The scientists were involved in the public health response (see the report Joining forces against the plague outbreak in Madagascar) with the Madagascan Health Ministry and the World Health Organization, and the outbreak was brought under control within a month.
Tackling Ebola in the Democratic Republic of the Congo
In spring 2018, an outbreak of Ebola hemorrhagic fever was reported in the Democratic Republic of the Congo (DRC). The current outbreak began in Equateur Province in the northwest of the country, a border region with the Republic of the Congo (Congo-Brazzaville). Although the first cases probably date from April 2018, the Ebola outbreak was confirmed in early May. "Ebola is known in the DRC and the symptoms were immediately suggestive of the disease," explains Prof. Arnaud Fontanet. "The diagnosis was made in a laboratory in Kinshasa. Once the results had been confirmed, there was a strong response from the national authorities. The World Health Organization also took rapid action." (See the article An update on Ebola in the Democratic Republic of the Congo.)
[video] What are the characteristics of the Ebola epidemic in the Democratic Republic of Congo? (source : Cnam)
Several scientists volunteered with the World Health Organization, which estimated the risk of spread of the virus in the DRC as very high. Staff at the Institut Pasteur and in the International Network responded via the Pasteur Outbreak Investigation Task Force (OITF) to the appeal made on May 17 by GOARN, WHO's Global Outbreak Alert and Response Network. Following WHO's concerted response to the outbreak, the focus is no longer on urban areas but on more isolated rural regions. A scientist from the Institut Pasteur, Amber Kunkel (from the OITF in the Center for Global Health directed by Arnaud Fontanet), arrived in the field to offer her epidemiological expertise to help tackle the outbreak. She arrived on Friday July 6 and traveled to Mbandaka, where she will work with the database of reported cases, help monitor alerts and draw up epidemiological reports. Her mission is currently due to last for four weeks. The OITF has also arranged regular information sessions with all Institut Pasteur staff working on the outbreak and, together with the International Affairs Department, members of the OITF have been working in conjunction with the partners and the Institut Pasteur International Network to provide remote support for the public health response.
Arnaud Fontanet explains: "This outbreak recently reached a turning point with the identification of a case on the outskirts of Mbandaka, an urban area with 1.2 million inhabitants. The risk of the disease spreading to built-up areas rekindles the fears of 2014, when the outbreak hit three West African capitals. So we urgently need to stop the virus spreading before it takes hold in towns and cities." According to WHO, the outbreak now appears to be under control. Once it is officially declared to be over, the OITF, the Center for Translational Science, the International Affairs Department and the International Network will turn their attention to how to improve anticipation of future Ebola outbreaks and support the Democratic Republic of the Congo in its plans for future research into Ebola (already discussed with WHO). The Institut Pasteur in Paris and five other members of the International Network in Sub-Saharan Africa are part of the ALERRT network (African Coalition for Epidemic Research, Response and Training). ALERRT, founded by the European & Developing Countries Clinical Trials Partnership (EDCTP), promotes clinical research on outbreaks, and the Institut Pasteur will be working with this network to develop future strategies.
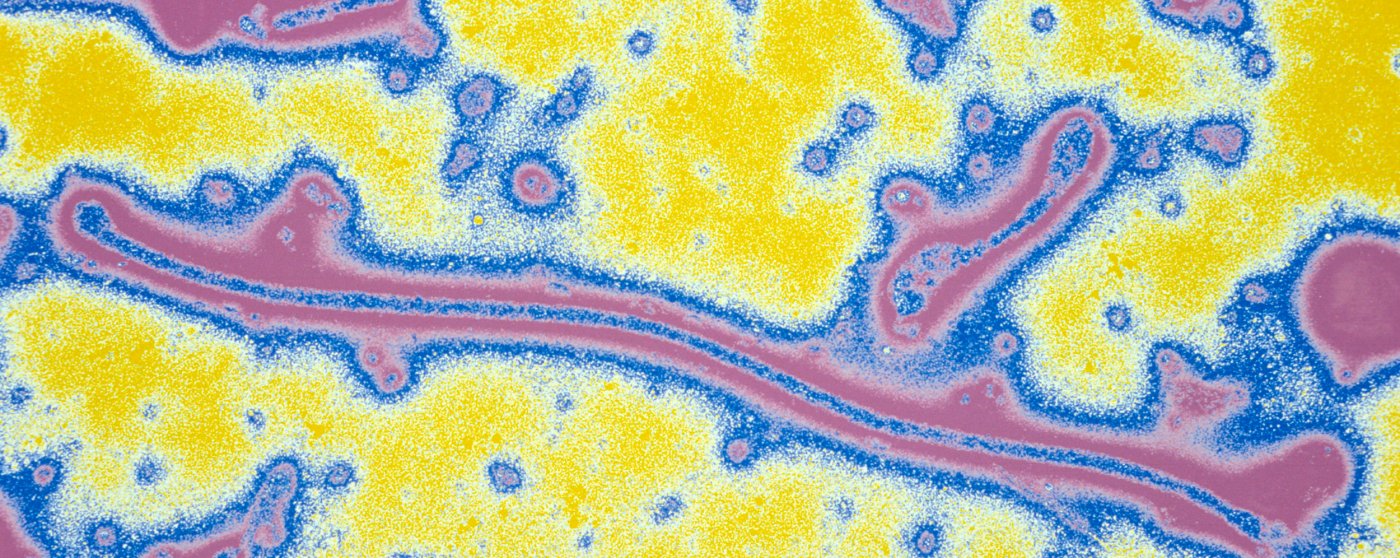
Ebola Virus (Filoviridae family). Filamentous virus (the longest known), responsible for severe fevers and internal haemorrhages often fatal for humans and monkeys. The reservoir of the virus would be the bat. Colorized image. © Institut Pasteur/Pierre Gounon
The Institut Pasteur offered its expertise
As well as the Epidemiology of Emerging Diseases Unit and the Center for Global Health directed by Arnaud Fontanet, other laboratories at the Institut Pasteur in Paris and in the International Network also responded via the OITF to the appeal from GOARN (the WHO Global Outbreak Alert and Response Network) and remain ready to step up if called on again by WHO.
● The Biology of Viral Emerging Infections Unit (UBIVE) directed by Sylvain Baize. This team has considerable expertise in the area of hemorrhagic fevers. It has access to the Inserm-Jean Mérieux BSL-4 laboratory, where the highly pathogenic Ebola virus can be handled. The National Reference Center for Viral Hemorrhagic Fevers at the Institut Pasteur, also directed by Sylvain Baize, is hosted in UBIVE. As the scientist explains: "The people sent out to the field are experts in their specialist areas who can help provide an effective response. We mustn't forget that a deployment means spending several weeks in the field in difficult conditions and maintaining a constant level of rigor when dealing with samples and analyzing data."
● CIBU (Laboratory for Urgent Response to Biological Threats), directed by Jean-Claude Manuguerra. This team can send several of its staff to help tackle outbreaks. The role of the CIBU is to offer an immediate response to biological emergencies by detecting and identifying a wide range of pathogens, both in France and abroad. As Jean-Claude Manuguerra, who has already been involved in previous missions, says: "We immediately responded to the call from WHO, because time always gives disease outbreaks the opportunity to spread. So we have to try to contain them as soon as possible. The Ebola virus is particularly dangerous and contagious because it spreads from person to person, so we need to diagnose cases effectively and quickly."
● In the International Network, the mobile laboratory run by the Institut Pasteur in Dakar was deployed in the field in Bikoro, and Prof. Gary Kobinger from Université Laval was approached to help the National Institute of Biomedical Research (INRB) in the DRC with field diagnosis.
Institut Pasteur staff have already been involved in a number of field missions, such as those organized during the world's largest ever Ebola outbreak in 2013-2016 (see the report Ebola 2013-2016: lessons learned and how to respond to new epidemics).
Animals, viral reservoirs
So where exactly do these emerging viruses come from? The first thing to note is that the term actually refers to several concepts:
- a "new", previously unknown virus that spreads throughout the human population. This was the case of the SARS coronavirus and also HIV, a major emerging virus of the 20th century.
- a newly identified virus that is responsible for a known disease. An example is the hepatitis C virus, which was discovered in 1989. This hepatitis virus was previously known as "non-A, non-B hepatitis". The agents responsible for many diseases remain unknown to this day.
- a known virus that spreads to new geographical areas, such as the Ebola virus, which occurred in West Africa in 2013-2016 after having previously only affected Central Africa. The West Nile, dengue and chikungunya viruses have also all spread to new geographical areas.
"New" viruses do not occur out of nowhere: as we saw with the SARS virus, they cross the "species barrier", spreading from animals to humans. AIDS, a disease discovered in the United States in 1981, had already been in existence for several decades in Africa, where the main virus responsible, HIV-1, is thought to have spread from monkeys to humans in the 1930s. The Ebola virus, responsible for a form of hemorrhagic fever that is fatal in 50 to 90% of cases, was first reported in 1976 in Sudan. It can be transmitted to humans by great apes, but its "reservoir" animals are bats.

A number of conditions must be met before "new" viruses spread from a limited geographical area to become a global threat (see interview below).

Every five years, humanity must deal with a major crisis caused by the emergence of a virus.
Can we predict emerging viruses?
When it comes to cancer, heart disease and neurodegenerative conditions, we can predict the number of cases that are likely to occur over the coming decades and make appropriate preparations. But with infectious diseases, it is impossible to plan ahead: everything can change in the space of one weekend, like in April 2009 when the entire world became aware of the new influenza virus that had emerged in Mexico. Epidemics have a considerable social and economic impact, as well as their more obvious repercussions on mortality and morbidity. Every five years on average, humanity must deal with a major crisis caused by the emergence of a virus. And this phenomenon will not disappear: the world's population is increasingly dense and increasingly mobile!
The conditions are ripe for an animal virus to emerge in humans, adapt to this new population and cause an epidemic.
How can a virus that has previously affected just a small number of people become a pandemic?
Everything depends on the first human cases. In the majority of cases, a virus that has managed to spread from animals to humans does not adapt to its new host, and the phenomenon disappears by itself. But random mutations can give rise to new variants that are better adapted to humans and are capable of spreading from one person to the next.
One example that illustrates this threat is the avian influenza virus, H5N1, which was able to infect approximately 600 people worldwide through direct contamination from poultry. Fortunately, H5N1, which is fatal in 50% of cases, cannot be transmitted from human to human. If this interhuman transmission were to become possible, even if H5N1 became less virulent in the process, we could be at risk of a major outbreak.
What can we do to prepare for such risks?
When it comes to disease outbreaks, time is precious: as more people are infected, the outbreak becomes more and more difficult to contain, the virus adapts increasingly well to humans and it may become even more contagious. Surveillance is being stepped up at global level so that we can rapidly detect clustered cases of patients displaying unusual symptoms and take action immediately, by adopting measures aimed at limiting the spread of these diseases such as isolation and quarantine. Then, using the first samples, it is important to identify the virus responsible as quickly as possible so that a diagnostic test can be developed. This is vital for managing the outbreak: it is crucial to identify which individuals are actually infected so that they and anyone they come into contact with can be treated. If we don't take action quickly, we soon lose control.
Preparing for emerging disease outbreaks is more important than ever
Recent examples of emerging disease outbreaks include SARS, H1N1 influenza, MERS-CoV, Ebola and Zika, all of which have had wide-ranging economic and public health consequences and contributed to social instability.
Rapid urbanization, greater mobility and global economic interdependence exacerbate the threat of emerging diseases and make containment all the more difficult. Moreover, 75% of the emerging infectious diseases that have affected humans over the last three decades are known to have a zoonotic origin. Human health is not only affected by an increasingly interconnected world; we now know that human health, animal health and the health of the surrounding ecosystem are inextricably linked. Being adequately prepared to detect, manage and respond to emerging outbreaks of infectious diseases is more important than ever.
The hundredth anniversary of the "Spanish" flu pandemic
"Towards the end of November 1918, Edmond Rostand left his house in Cambo-les-Bains and headed to Paris to join in the festivities and soak up the celebratory atmosphere following the end of the First World War. One evening he went to the Sarah Bernhardt Theater to watch a rehearsal of L'Aiglon starring the great actress. He caught a chill in the wings and went home shivering and suffering from chest pains. The next day, November 30, his temperature reached 41°C. Two days later he was dead."
The sudden demise of the author of Cyrano de Bergerac illustrates the violence of the "Spanish" flu, which also claimed the lives of French poet Guillaume Apollinaire, Austrian painter Egon Schiele and 40 million others worldwide (more than 400,000 in France alone).
This influenza outbreak was more deadly than the First World War, spreading over the entire planet in the space of a few months and affecting more than a third of the global population between 1918 and 1919. It was referred to as "Spanish flu" because Spain, a neutral country and therefore not bound by military secrecy in wartime, was the first country to mention the epidemic publicly.
But it is actually thought that this pandemic, the deadliest in history over such a short space of time, had its origins in China, before spreading to the United States through a US battalion returning from Guangzhou to a base in Boston. The first deaths were reported in US military camps in February 1918. The virus is believed to have mutated in the United States, becoming more virulent and deadly, and then to have been introduced in Europe that spring with the arrival of American troops. It soon spread throughout the rest of the world via trade between the European powers and their colonies.
The 1918 A(H1N1) virus (a remote ancestor of the virus that caused the 2009 pandemic) was characterized in the 1990s by Jeffery Taubenberger's team, which analyzed tissue samples preserved in paraffin from collections belonging to the US military health service, taken from two soldiers who died in 1918. The same team then revealed the whole genome sequence of the virus in 2005, using viral RNA isolated from the lungs of an Inuit woman, another victim of the 1918 pandemic, who was exhumed from the Alaskan permafrost (deep layers of soil or rock that remain frozen all year round) by Swedish pathologist Johan Hultin. Although we now understand a lot more about the pandemic, we still do not know why the 1918 virus was so virulent. Elucidating the mystery would help us to evaluate the risks associated with other strains of influenza. The scientific investigation goes on...

"What a strange taste to your cake!
- An antipyrine bean, because of the Influenza! "
The first pandemic of the 21st century
Metropole Hotel, Hong Kong, room 911 on the ninth floor: Dr. F. arrived from mainland China. He wasn't feeling well. He was experiencing similar symptoms to some patients that he had recently treated in Guangdong province. He had a cough and a headache, breathing difficulties and a high temperature. He was admitted to hospital the next day after coming into contact with several people in the reception, lifts and corridors of the Metropole Hotel. Mrs. L., a 78-year-old Canadian tourist, was staying at the hotel at the same time. When she returned to Canada a few days later, she met up with her family, little realizing that this gathering would have devastating consequences.
At the same time, a Chinese-American businessman was admitted to the French Hospital of Hanoi, Vietnam, suffering from "atypical pneumonia". Then a young woman aged 26 arrived at the hospital with the same symptoms. What did these two patients have in common? They had both stayed on the ninth floor of the Metropole Hotel. Dr. F. soon died in Hong Kong. Five members of Mrs. L.'s family fell ill in Toronto, triggering an outbreak in Canada. At the French Hospital of Hanoi, 22 members of staff were found to be suffering from "atypical pneumonia". In Singapore, an outbreak of the mysterious disease could also be traced back to guests at the Metropole Hotel. All these events took place within just two weeks.
Is this the scenario of a science fiction movie?
No, this sequence of events really happened, between February 21 and March 10, 2003, shortly before the World Health Organization launched a global alert in a bid to contain what would be the first emerging disease outbreak of the 21st century: SARS, or severe acute respiratory syndrome. WHO was aware that a strange contagious disease had been witnessed in the Chinese province of Guangdong over the past few weeks. And when guests at the Metropole Hotel became infected, the outbreak spread beyond China's borders. Within weeks, the cause of SARS was identified as a previously unknown coronavirus. Himalayan masked palm civets, animals whose meat was enjoyed as a delicacy in some provinces of China, were suspected of having transmitted the virus to humans. Trade in these animals was banned and farms were destroyed. Restrictions on international flights to the affected areas, isolation of contaminated individuals, enforced quarantine for their contacts and collaboration among laboratories worldwide meant that the outbreak was able to be contained within a few months – but not before it had affected some 8,000 people and claimed nearly 800 lives in 27 countries. As well as the lives lost, SARS had a major economic impact – the overall cost of the outbreak is estimated at €25 billion.
What happened at the Metropole Hotel in Hong Kong – where one "super-spreader" was responsible for the coronavirus traveling to several countries – clearly shows how easily a new virus can spread rapidly across the entire planet. Any virus that emerges anywhere in the world is, after all, no more than a flight away from us.

The global health mission of the Institut Pasteur and the Institut Pasteur International Network
All deployments by volunteers are coordinated at the Institut Pasteur by the Outbreak Investigation Task Force (OITF).
The OITF was set up in 2014 during the first major Ebola outbreak. In 2015, more than 50 scientists from 10 institutes in the Institut Pasteur International Network joined the task force. The OITF is now open to all scientists in the International Network.
Members of the OITF represent a wide variety of disciplines including epidemiology, microbiology, entomology, social science and veterinary medicine. Every year, OITF members, especially from the Institut Pasteur in Paris, are sent to tackle various outbreaks worldwide, in conjunction with WHO and in support of the institutes in the International Network.
Via the OITF, scientists from the Institut Pasteur and the International Network are eligible to be deployed with WHO to help tackle health crises, whether as part of multilateral responses (coordinated by WHO) or bilateral responses (to support other institutes in the International Network), or directly at the invitation of a country's government.
Eileen Farnon, OITF Coordinator, points out that "My role, as coordinator of the task force, is to work directly with WHO and other partners so that we can provide the quickest and most effective response to the medical needs of populations affected by an outbreak. I notify all the members of the Institut Pasteur in Paris and in the International Network of any calls for assistance made by GOARN. We have a very strong network and it is our responsibility to select the best experts to tackle large-scale health crises and support Health Ministries as they deal with outbreaks. To monitor the latest Ebola outbreak in the DRC, the OITF selected specialists from the Institut Pasteur in Paris and Lyon and from other institutes in the International Network (Algeria and Shanghai). Providing an effective response to global outbreaks often requires the help of international institutions, which can shed light on the nature of the disease and help bring it under control, facilitating prevention and detection and improving the response to any future outbreaks." The Institut Pasteur has committed scientists who can support countries in these multidisciplinary areas, either in partnership with organizations such as GOARN and ALERRT or directly in the field. "The OITF facilitates this support from the Institut Pasteur in Paris and from the Institut Pasteur International Network. It simplifies communication within the network to help countries by providing an effective, coordinated response to outbreaks."
Within the Institut Pasteur International Network, a number of institutes have demonstrated their ability to work effectively alongside national and international health authorities, particularly in improving access to diagnosis. As Marc Jouan, Director of the International Network, emphasizes: "During the last outbreak, the Institut Pasteur institutes in Africa showed that they are capable of making a long-term contribution to help tackle this type of health crisis and cooperate with neighboring countries."



