The aim of immunotherapy strategies is to use cells in the patient's own immune system to destroy tumor cells. CAR T cell therapy is an immunotherapy that is effective in treating blood cancer. Around 35,000 people are affected by blood cancer each year in France, with 1.24 million cases worldwide. By closely investigating some of the immune cells generated during this therapy, known as CD4 T cells, scientists from the Institut Pasteur and Inserm, in collaboration with clinicians from the Paris Public Hospital Network (AP-HP), discovered that these cells are capable of remotely neutralizing tumor cells by producing interferon gamma (IFN-γ). This study raises new hopes for blood cancer patients with an incomplete response to CAR T cell therapy and for cancers sensitive to IFN-γ. The results were published on May 29, 2023 in the journal Nature Cancer.
CAR T cell therapy is an immunotherapy that has produced remarkable results in treating certain types of leukemia or lymphoma. But some patients who receive this treatment relapse because their tumor cells evade the therapy. A multidisciplinary team of scientists from the Institut Pasteur and Inserm and clinicians from the Paris Public Hospital Network (AP-HP) sought to shed light on how the therapy works with the aim of obtaining even more effective responses.
The principle of CAR T cell therapy is to isolate the patient's T cells, genetically modify them so that they specifically target tumor cells, and multiply them before injecting them back into the patient in large numbers. This army of killer CAR T cells is composed of CD4 T cells and CD8 T cells in varying proportions from one patient to the next. While we know that CD8 killer T cells need to come into direct contact with tumor cells to destroy them, the mechanism of action of CD4 T cells had not previously been fully explored.
The research team studied these CD4 CAR T cells more closely and revealed a very interesting property: their ability to kill tumor cells remotely by secreting a molecule involved in the immune response, interferon gamma (IFN-γ).
"For some types of cancer that are sensitive to IFN-γ, this destruction mechanism is highly efficient. We also observed that, among patients with a high quantity of CD4 T cells, those that produce a large amount of IFN-γ respond better to treatment," explains Philippe Bousso, Head of the Institut Pasteur's Dynamics of Immune Responses Unit (Inserm 1223) and last author of the study.
To reveal the novel mechanism of action of these remote killer cells, the scientists began by exploring preclinical models to analyze the mechanism in detail, in particular using in vivo imaging techniques, then they checked the relevance of the results on samples from patients.
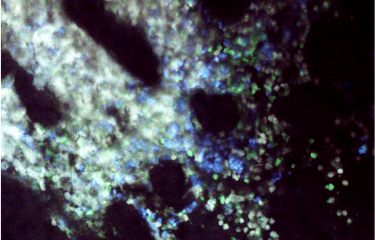
CAR T cells attacking a tumor, visualized using intravital imaging. Live tumor cells are shown in white, apoptotic tumor cells in blue and CAR T cells in green. The white circles indicate the destruction of tumor cells when they come into direct contact with CAR T cells, and the red circles show the remote destruction of tumor cells. © Morgane Boulch, Philippe Bousso / Institut Pasteur
"This discovery opens new avenues for adjusting treatments to prevent tumor cells evading CAR T therapy. It also raises new therapeutic hopes for patients, offering the possibility of a more personalized treatment approach whereby a larger quantity of CD4 CAR T cells can be used to activate IFN-γ depending on tumor cell sensitivity," comments Philippe Bousso.
By developing a better understanding of how CD4 T killer cells work, it may also be possible to extend the scope of this therapy to other solid tumor cancers that are sensitive to IFN-γ. The clinical data will be confirmed on other cohorts.
This research was funded by the institutes cited above, and also by the French National Cancer Institute (INCa) and the European Research Council.
Source
Tumor-intrinsic sensitivity to the pro-apoptotic effects of IFN-γ is a major determinant of CD4+ CAR T-cell antitumor activity, Nature Cancer, May 29, 2023
Morgane Boulch1, Marine Cazaux1, Alexis Cuffel 2,3, Marion V. Guerin1, Zacarias Garcia1, Ruby Alonso1, Fabrice Lemaître1, Alexander Beer1, Béatrice Corre1, Laurie Menger4, Capucine L. Grandjean1, Florence Morin2, Catherine Thieblemont5, Sophie Caillat-Zucman2,3 & Philippe Bousso1
1 Institut Pasteur, Université de Paris Cité, INSERM U1223, Equipe Labellisée Ligue Contre le Cancer, Paris, France.
2 Université de Paris Cité, Hôpital Saint-Louis, AP-HP Nord, Laboratoire d’Immunologie, Paris, France.
3 INSERM UMR976, Institut de Recherche St-Louis, Paris, France.
4 Gustave Roussy, Villejuif, France ; INSERM U1015, Villejuif, France.
5 Service d’Hémato-Oncologie, Hôpital Saint-Louis, AP-HP, Université de Paris Cité, Paris, France.