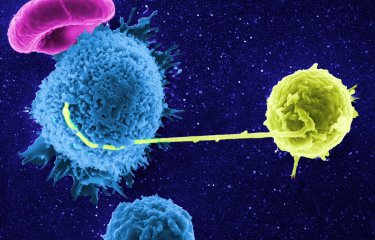
HIV controllers are rare individuals able to control infection naturally without treatment. CD8+ T immune cells play a critical role for these individuals, suppressing viral load in the long term even without antiretroviral therapy. Scientists at the Institut Pasteur are examining key characteristics of these controllers' CD8+ T cells, with a view to replicating them in other individuals who are incapable of controlling the virus without treatment. They have successfully reprogrammed CD8+ T cells from non-controllers and bestowed them with properties of controllers' cells. Cell reprogramming was performed in vitro by temporarily exposing them to a molecule that targets signaling pathways mobilized within controllers' cells. These findings, published on April 5, 2022 in The Journal of Clinical Investigation, provide proof of concept for a cell therapy that could prove effective in achieving HIV remission. .
HIV controllers are rare individuals identified as being able to control viral infection naturally without treatment. In these very rare individuals (less than 1% of people living with HIV) no multiplication of the virus in the blood can be detected after more than 10 years of infection without treatment. In 2007, scientists at the Institut Pasteur described how, unlike non-controllers' CD8+ T cells, those of controllers are able to rapidly destroy infected CD4+ T cells. Asier Sáez-Cirión's group also demonstrated in a previous study that the cells from controllers use a different molecular program. Their research shows that anti-HIV CD8+ T cells in controllers not only have huge antiviral potential; they are also programmed to survive, whereas in non-controllers, the cell program predisposes them to exhaustion and cell death.
In continuation of this work, the scientists at the HIV, Inflammation and Persistence Unit at the Institut Pasteur have now successfully reprogrammed non-controllers' CD8+ T cells to acquire key characteristics of controllers' cells, namely their ability for memory, survival, expansion, resistance to exhaustion, and polyfunctionality including an enhanced ability to suppress HIV infection. The ability of CD8+ T cells to acquire and maintain such properties appears crucial to achieving natural control of HIV.
This reprogramming was performed in vitro through temporary exposure of HIV non-controllers' cells to a GSK 3 inhibitor, a small molecule targeting two signaling pathways identified as essential to optimal functioning of CD8+ T cells. The scientists observed in vitro that this reprogramming promoted functional capacities associated with natural control of infection.
"The goal of this study is to eventually use this strategy as part of cell therapy to achieve HIV remission. This would involve isolating non-controllers' cells, reprogramming them ex vivo, and re-injecting them before potentially discontinuing treatment," commented Asier Sáez-Cirión, Leader of the Viral Reservoirs and Control Group at the Institut Pasteur and coordinator of the study.
Moreover, there may be applications beyond HIV for these findings, since the cellular characteristics achieved after reprogramming are highly desirable for cancer cell therapies.
This study was conducted in collaboration with the ANRS CO6 PRIMO and CO21 CODEX cohorts and the Paris Public Hospital Network (AP-HP), with funding from ANRS | Emerging Infectious Diseases and the NIH ((1UM1AI164562-01).
Source :
Reprogramming dysfunctional CD8+ T cells to promote properties associated with natural HIV control, The Journal of Clinical Investigation, 5 avril 2022
Federico Perdomo-Celis1, Caroline Passaes1, Valérie Monceaux1, Stevenn Volant2, Faroudy Boufassa3, Pierre de Truchis4, Morgane Marcou4, Katia Bourdic5, Laurence Weiss6, Corinne Jung6, Christine Bourgeois5, Cécile Goujard7, Laurence Meyer3, Michaela Müller-Trutwin1,Olivier Lambotte5, and Asier Sáez-Cirión1,*
1Institut Pasteur, Université Paris Cité, Unité HIV Inflammation et Persistance, F-75015 Paris, France.
2Institut Pasteur, Université Paris Cité, Hub Bioinformatique et Biostatistique, F-75015 Paris, France.
3Université Paris Saclay; Inserm CESP U1018; APHP, Department of Public Health, Bicêtre Hospital, Paris Saclay, France.
4Université Paris-Saclay, AP-HP Hôpital Raymond Poincaré, Garches, France
5Université Paris-Saclay, AP-HP, Bicêtre Hospital, UMR1184 INSERM CEA, Le Kremlin Bicêtre, France.
6Université de Paris Cité, AP-HP, Paris Centre, Hôtel Dieu, Paris, France
7Université Paris-Saclay, AP-HP, Hôpital Bicêtre, DMU 7, Inserm U1018, CESP, 94290, Le Kremlin Bicêtre, France.
*Corresponding author