When a bacterium invades a host, it seeks to proliferate by multiplying. Bacterial cell division is coordinated by several mechanisms including the synthesis of peptidoglycan, a thick complex surrounding the cell. This synthesis is enabled by the action of a specific enzyme (lytic transglycosylase). Scientists from the Biology and Genetics of the Bacterial Cell Wall Unit at the Institut Pasteur have discovered a region of this enzyme that opens a chink in the bacterial armor when subjected to genetic interference. Thanks to the discovery, this enzyme can now be considered as a target for treatments against infections by causative agents.
Bacterial cell division is regulated by the synthesis of peptidoglycan, a large, complex "chain mail shirt" surrounding cells. It determines the shape, rigidity and survival of bacteria. Elements on the cell surface also cling to it. Peptidoglycan formation is dependent on the combined efforts of two molecular enzymatic machineries. One of these contains lytic transglycosylase present in all gram-negative bacteria, and enables an immune response to be triggered to infection.
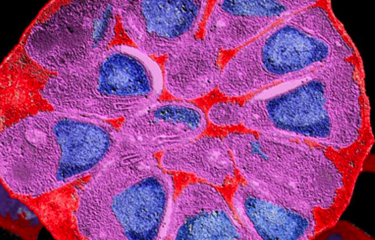
Enzymatic reactions in Neisseria meningitidis
In order to clarify the role played by this enzyme in bacterial physiology and assess its potential as a target for antimicrobial treatments, scientists at the Institut Pasteur Biology and Genetics of the Bacterial Cell Wall Unit captured a high-precision image showing enzymatic reactions of lytic transglycosylase in the bacterium Neisseria meningitidis (see fact sheet on meningitis). This bacterium was selected for study as it is a major human causative agent providing a template for its close relative gonococcus, with both bacteria growing increasingly resistant to antibiotics.
Weakened bacterial machinery
"We identified an area of interest in the enzyme which, when subjected to genetic interference, compromises the integrity of the cell wall and also affects the bacterium's ability to divide and proliferate," explains Allison Williams, a scientist with the Biology and Genetics of the Bacterial Cell Wall Unit at the Institut Pasteur. Drawing on experience gained from the study, it has been possible to reduce the virulence of Neisseria meningitidis during infection.
"We also demonstrated that such genetic interference leads to significant modifications of peptidoglycan, thus altering the functions of certain partner proteins of the enzyme lytic transglycosylase," adds Ivo G. Boneca, head of the Biology and Genetics of the Bacterial Cell Wall Unit at the Institut Pasteur. Disruption of these partner proteins also helps to weaken the bacterial cell wall.
A long-term collaborator, Dr. Muhamed-Kheir Taha, head of the Invasive Bacterial Infections Unit at the Institut Pasteur concludes: "The reduction in meningococcal virulence observed in this multidisciplinary study may lead to significant progress in the search for new antimicrobial therapies targeting this enzyme."
This study therefore demonstrates the importance of targeting specific areas of the bacterial molecular machinery, potentially preventing interaction with its partners and thus paving the way to new antimicrobial treatments.
Source
Defective lytic transglycosylase disrupts cell morphogenesis by hindering cell wall de-O-acetylation in N. meningitidis, eLife, 2020 February, 5
Allison H. Williams1*, Richard Wheeler1,2, Ala-Eddine Deghmane3, Ignacio Santecchia1,4, Ryan E. Schaub5, Samia Hicham1, Maryse Moya Nilges6 Christian Malosse7, Julia Chamot-Rooke7, Ahmed Haouz8, Joseph P. Dillard5, William P. Robins9, Muhamed-Kheir Taha3, Ivo Gomperts Boneca1*
Affiliations
1Unité Biologie et Génétique de la Paroi Bactérienne, Institut Pasteur; Groupe Avenir, Inserm, 75015 Paris, France
2Tumour Immunology and Immunotherapy, Institut Gustave Roussy, F-94805 Villejuif, France
3Unité des Infection Bactériennes Invasives, Institut Pasteur, 75015 Paris, France
4Universté Paris Descartes, Sorbonne Paris Cité, Paris, France
5Department of Medical Microbiology & Immunology, University of Wisconsin-Madison, Madison, WI, USA.
6Unité Technologie et Service BioImagerie Ultrastructural, Institut Pasteur, 75015, Paris, France
7Unité Technologie et Service Spectrométrie de Masse pour la Biologie, Institut Pasteur; UMR 3528, CNRS, 75015 Paris, France
8Plate-forme de Cristallographie, Institut Pasteur; UMR3528, CNRS, 75015 Paris, France
9Department of Microbiology, Harvard Medical School, Boston, MA, USA.
*Correspondence to: Allison H. Williams, e-mail: awilliam@Pasteur.fr / Ivo G. Boneca, e-mail: bonecai@Pasteur.fr
This study is part of the priority scientific area Antimicrobial Resistance of the Institut Pasteur's strategic plan for 2019-2023.