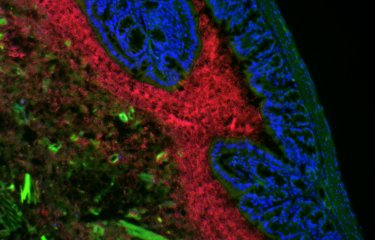
Researchers at the Institut Pasteur (Paris) recently combined fluorescence imaging, computational modeling, and electron microscopy to show how the diversity of nanoscale protein assemblies drives diversity in communication between neurons in the brain. This study opens up new avenues of research for linking molecular function to alterations in information processing by the brain, and may also serve as a building block for understanding alterations in brain connectivity diseases such as autism, schizophrenia and depression.
Communication between neurons in the brain occurs through synapses. Synaptic strength and plasticity are fundamental parameters for routing information throughout the brain and storing memories. Unlike electrical connections within manufactured electrical circuits, “synapses are diverse in terms of their efficiency of communication (synaptic strength), and are extremely plastic”, explains David DiGregorio, Head of the Synapse and Circuit Dynamics Laboratory at the Institut Pasteur (CNRS – UMR3571). This diversity in information flow is partly what makes our brain a unique and powerful computing device.
Nanoscale organization between proteins at synapses is necessary for driving communication between chemical synapses
Researchers at the Institut Pasteur studied the structural organization of synaptic proteins and their influence on the strength of interneuronal communication. Ion channel proteins, for example, are special arrangements of amino acids which embed in the cell membrane (in this case the neuron membrane), and provide passageways for small, polar ions, such as Ca2+ (calcium ions). Calcium is a universal signaling molecule in all cells, and for synapses in the brain is responsible for the release of chemical neurotransmitters from synaptic vesicles. Neurotransmitters are the currency for communication between two neurons connected by chemical synapses. However, the proximity between calcium channel proteins and the synaptic vesicle proteins on which Ca2+ act is critical for regulating the strength and precision of communication. “Thus by studying the nanoscale organization of synaptic proteins we can decipher the molecular code regulating different types of synaptic communication,” sums up David DiGregorio.
Synaptic proteins diversity drives synaptic strength and neuromodulation diversity
Until recently, scientists knew that the distance between synaptic proteins must be important, but had little information about the actual two-dimensional nanoscale topographical arrangement of calcium channels and synaptic vesicles, and how it related to the strength and efficiency of synaptic communication. “We hypothesized that the different spatial distribution of these proteins was related to the diversity of synaptic function.” David DiGregorio’s team recently combined fluorescence imaging, computational modeling, and electron microscopy, showing in particular that the number of presynaptic calcium channels (CaV) does not correlate with synaptic strength, and that different nanoscale CaV-synaptic vesicle arrangements explain functional differences. “For the first time we show how diversity in the two-dimensional nanotopography of synaptic proteins drives diversity of synaptic strength and its differential modulation by pharmacological compounds.”
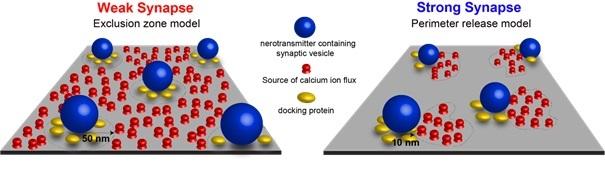
This study opens up new avenues of research for linking molecular function to alterations in information processing by the brain, and may also serve as a building block for understanding alterations in brain connectivity diseases such as autism, schizophrenia and depression.
Source
Distinct Nanoscale Calcium Channel and Synaptic Vesicle Topographies Contribute to the Diversity of Synaptic Function, Neuron, November 20, 2019.
Nelson Rebola 1 4 5, Maria Reva 1 5, Tekla Kirizs 2, Miklos Szoboszlay 2 3, Andrea Lőrincz 2, Gael Moneron 1, Zoltan Nusser 2, David A. DiGregorio 1 6
1. Institut Pasteur, unité Imagerie dynamique du neurone, CNRS UMR 3571, Paris, France
2. Laboratoire de neurophysiologie cellulaire, Institut de médecine expérimentale, Académie hongroise des sciences, Budapest, Hongrie
3. Adresse actuelle : Mortimer B. Zuckerman Mind Brain Behavior Institute, Department of Neuroscience, Columbia University, New York, NY, États-Unis
4. Adresse actuelle : Institut du cerveau et de la moelle épinière (ICM), Hôpital Pitié-Salpêtrière, Sorbonne Universités, Inserm, CNRS, Paris 75013, France
5. Ces auteurs ont contribué à part égale.
6. Contact principal
This study is part of the priority scientific area Brain connectivity and neurodegenerative diseases of the Institut Pasteur's strategic plan for 2019-2023.