Epidemiological data have already revealed a link between obesity and chronic inflammatory bowel disease. Given the increasing prevalence of obesity worldwide, it is vital to elucidate the mechanisms by which obesity can lead to complications. Scientists in the Microenvironment and Immunity Unit at the Institut Pasteur recently demonstrated that a high-fat diet or over-eating early in life significantly increases the risk of developing inflammatory diseases. Understanding this "disease imprint", which remains in the body after infancy, is a first step towards future research that may result in the development of preventive measures.
Obesity, especially childhood obesity, is a known risk factor for developing metabolic disorders in adulthood and related conditions such as atherosclerosis, high blood pressure, type 2 diabetes and fatty liver disease.
Scientists have identified several potential links between obesity and a predisposition to inflammatory diseases:
- the transfer and development of an imbalanced microbiota;
- epigenetic modifications;
- mutations in genes responsible for controlling inflammation.
Previous work by scientists in the Microenvironment and Immunity Unit had already demonstrated in animal models that a key immune response is generated when solid food is introduced and the microbiota develops. "We showed that this immune reaction is vital because it helps educate the immune system and leads to low susceptibility to inflammatory diseases in adulthood," explains Gérard Eberl, who leads the unit at the Institut Pasteur. But little was known about the impact of infant nutrition on the immune system and the consequences for future health.
A first step towards understanding the "disease imprint"
In new research published recently in Nature Metabolism, the scientists again used animal models to demonstrate that exposure to over-eating before weaning may increase intestinal permeability and the expression of inflammatory molecules known as cytokines. "We demonstrate that over-eating and a high-fat diet "feeds" some bacteria, breaks links between proteins [known as "disulfide bonds"] and causes an imbalance in the gut mucosa," explains Gérard Eberl. These molecular disruptions result in increased long-term susceptibility to colitis. The scientists also showed that this susceptibility can be overcome by preventing such disruptions.
These findings are a first step to further research: "We now need to identify what type of food in particular has an impact, define a dietary threshold and identify the bacteria responsible for these disruptions so that we can differentiate between physiological and pathological aspects," explains Gérard Eberl.
All of these data point towards the existence of a "disease imprint". "It is interesting to note that mechanisms which occur very early on in life have a long-term influence on health. The body has a sort of memory. We now need to find out whether it is possible to correct it," concludes Gérard Eberl
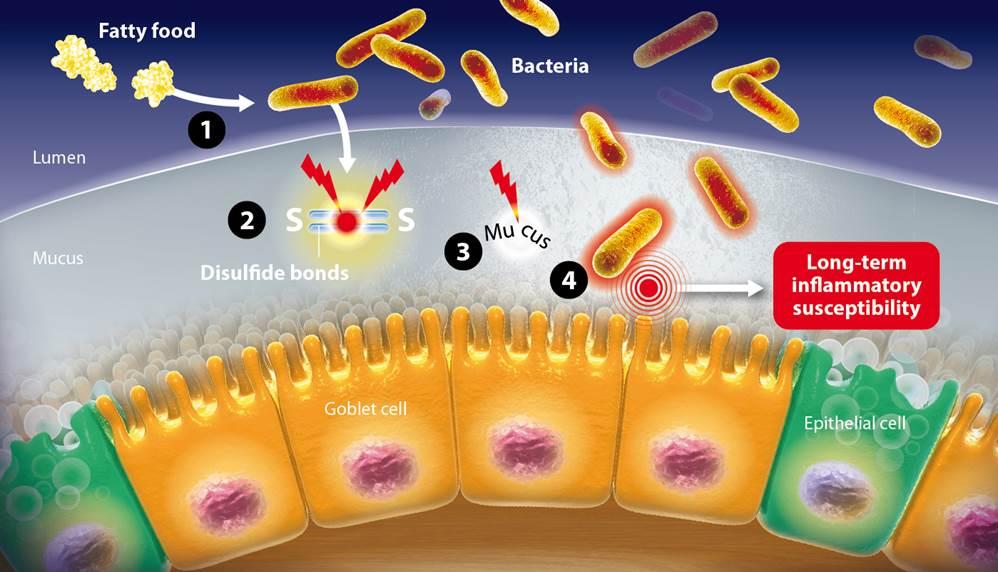
Captions of the illustration:
1- The fatty food allows bacteria to proliferate
2- The disulfide bonds are broken by bacteria
3- The intestinal mucus is imbalanced
4- Bacteria penetrate the mucus
5- This inflammation has a long-term influence on the immune system
Source
Excess calorie intake early in life increases susceptibility to colitis in adulthood, Nature Metabolim, November 4th, 2019
Ziad Al Nabhani1,2, Sophie Dulauroy1,2, Emelyne Lécuyer1,2, Bernadette Polomack1,2, Pascal Campagne3, Marion Berard4 and Gérard Eberl1,2
1 Institut Pasteur, Microenvironment & Immunity Unit, Paris, France
2 INSERM U1224, Paris, France
3 Hub de Bioinformatique et Biostatistique, Département de Biologie Computationnelle, Institut Pasteur, USR 3756 CNRS, Paris, France
4 Institut Pasteur, DTPS, Animalerie Centrale, Centre de Gnotobiologie, Paris, France