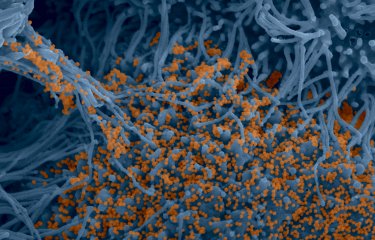
SARS-CoV-2, COVID-19 epidemic: the Institut Pasteur's response, scientific research and discoveries in 2020
The Institut Pasteur is one of the world's leading centers for research on infectious diseases. This area is therefore one of the priority research areas of its 2019-2023 strategic plan. On January 27, 2020, a 'coronavirus task force' was set up to respond to the urgency of this health crisis, by studying the virus and the disease it causes - the task force was created at a time when the 11 million inhabitants of Wuhan (China) are 'locked down' -. Here is a report summarizing the events and reviewing the Institut Pasteur's main action.
The information in this report is dated from December 31, 2020.
On December 31, 2019, the China Country Office of the World Health Organization (WHO) was informed that clustered cases of pneumonia of unknown etiology had been detected in the city of Wuhan, in the Chinese province of Hubei. On January 9, 2020, the Chinese health authorities and WHO announced the discovery of a novel coronavirus, thought to have emerged in Wuhan in December 2019, that was responsible for the outbreak. The date of emergence of the virus in China was subsequently called into question. The coronavirus, named SARS-CoV-2 on February 11, was identified as the agent responsible for a new infectious respiratory disease subsequently named COVID-19 (coronavirus disease 2019). This officially marked the start of an epidemic that would rapidly spread across the globe and would be classified as a pandemic by WHO on March 11, 2020.
Institut Pasteur at the heart of the national response in France
The Institut Pasteur found itself on the front line of the response in France from the very start of the epidemic, in January 2020. The Institut Pasteur hosts several National Reference Centers (CNRs), designated by the French General Directorate of Health and Santé publique France, which are responsible for monitoring various infectious diseases. These include the CNR for Respiratory Infection Viruses (including Influenza), which had the task of diagnosing, monitoring and analyzing cases of COVID-19 in mainland France as soon as the first cases were suspected. The Laboratory for Urgent Response to Biological Threats (CIBU), set up in 2001 by the Institut Pasteur with the support of the General Directorate of Health so that it could intervene 24/7 in the event of an outbreak, was immediately mobilized to strengthen the work of the CNR. The first suspected cases in France were identified on January 24, 2020 (source: French Ministry of Health), and the samples taken from these patients were analyzed by the CNR, which confirmed infection with the SARS-CoV-2 virus that by now was sweeping through China, based on the sequence that Chinese scientists had shared with the international scientific community.
Task force rapidly set up
The Institut Pasteur is a leading global center for infectious disease research, one of the priority research areas identified in its 2019-2023 Strategic Plan. On January 27, 2020, as the 11 million inhabitants of the Chinese city of Wuhan, where COVID-19 had originated, found themselves in a strict lockdown, the Institut Pasteur set up a task force to provide an emergency response to the health crisis by studying the virus and the disease it causes. The task force continues to draw on the expertise of Institut Pasteur scientists in several research fields:
- Knowledge of the virus and its pathogenesis;
- Development of new diagnostic and serological tools;
- Research into therapeutic strategies, including antibody-based approaches;
- Vaccine development;
- Epidemiology and modeling to develop outbreak control strategies.
Around 20 research projects were launched in late January, and 89 projects in total over the whole of 2020.
This document was first completed between November and December 2020 and subsequently finalized in January 2021 in the current unprecedented COVID-19 pandemic. Continuing and untiring efforts to fight SARS-CoV-2 result in a workload increase for many Institut Pasteur teams.
We wish to extend our heartfelt thanks to all contributors for their commitment in these challenging times and to the readers of this "COVID-19 special" for their understanding when reading these pages.
"On January 25, 2021, we were ready to share the fruits of the Institut Pasteur's mobilization in 2020 against Covid-19. The document you are reading has been prepared since last november by our teams, scientists and administrative staff, all highly mobilised. And, on this same 25 January, the Pasteur Institute had to take a serious decision, with sadness and responsibility: to interrupt one of its programmes of candidate vaccines against SARS-CoV-2. Interim Phase I clinical trial results were half-toned. It was the only scientific decision to be taken, with humility.
We started to communicate our results at the end of January but I wanted to take over the a few days after the event, as a reminder that modesty and perseverance are virtues, for any self-respecting scientist. They have also been Pasteurian values for more than 130 years, for the benefit of human health.
We are therefore pursuing with determination the development of other candidate vaccines that arrived in end of the pre-clinical phase, and maintaining our mobilization to fight the Covid-19 epidemic. Because we have done a great deal of work in terms of diagnosis to identify patients, of modelling to monitor the epidemic, or of basic research to understand the virus.
During such an eventful, turbulent year, it was important for us to remained united and committed. I was both impressed and extremely touched, as were all the Institut Pasteur teams, by the incredible resilience of citizens in France and worldwide as they were confronted with this very brutal epidemic. I was also impressed by the solidarity and generosity shown by each and every individual, many of whom went out of their way to make masks, support healthcare workers, help isolated neighbors and show their support for research and scientists.
Driven by this collective momentum, in sometimes difficult working conditions – as was the case for everyone –, the Institut Pasteur carried out extensive research on COVID-19 and the SARS-CoV-2 virus which causes the disease, with the support of its partners from hospitals, academia and industry. I would like to thank in particular the caregivers who have been at our side for the many clinical studies launched in 2020. Thanks to them, and to you, we have been responsive and have initiated a very large number of projects, much more than we thought. We will continue to act, together, with unwavering dedication!"

Stewart Cole
President of the Institut Pasteur (Paris)
Our mobilization is unprecedented. Our experts in many disciplines are helping to provide answers to this serious health crisis, and some research will be pursued over the long term.
 THE FIRST DIAGNOSTIC TEST FOR PATIENTS
THE FIRST DIAGNOSTIC TEST FOR PATIENTS

In the second half of January, the National Reference Center (CNR) for Respiratory Infection Viruses, which has the general task of monitoring infectious diseases, developed a direct detection test for the coronavirus using a molecular biology method known as RT-qPCR.
This test was used to diagnose the first patients in France and was then rolled out in hospitals, serving as a benchmark for the development of other PCR tests in France. In 2020, the Institut Pasteur also developed an RT-LAMP test for rapid diagnosis in 10 to 30 minutes.
See Progress in diagnostics and epidemiological genomics
 PHYLOGENETIC ANALYSES AND SEROLOGICAL ASSAYS TO DESCRIBE AND MAP THE EPIDEMIC
PHYLOGENETIC ANALYSES AND SEROLOGICAL ASSAYS TO DESCRIBE AND MAP THE EPIDEMIC

Phylogenetic analyses, like the one performed by the Institut Pasteur in early 2020 on around a hundred patient genomes, help scientists to understand and describe how a virus is introduced in a geographic region (such as France), and how it first circulates. The Institut Pasteur also developed various serological assays, which detect SARS-CoV-2 antibodies in the blood and determine whether an individual has been infected by the virus in the preceding weeks.
These serological assays are used to map the spread of the virus in the population, via seroprevalence studies in a given region (in Crépy-en-Valois, at national level, etc.) or cohort monitoring (e.g. among hospital/non-hospital staff from the Institut Curie and the Institut Pasteur in the Curie-O-SA study).
 MODELING TO VISUALIZE THE EVOLUTION AND PROGRESSION OF THE EPIDEMIC
MODELING TO VISUALIZE THE EVOLUTION AND PROGRESSION OF THE EPIDEMIC

How can we best predict the evolution of the epidemic situation? How can we anticipate the admission of severely ill patients to hospital? Drawing on its expertise in the mathematical modeling of infectious diseases, the Institut Pasteur developed tools to analyze the spread of the epidemic in real time, week after week. The data are regularly updated to improve the accuracy of possible evolutionary scenarios.
See Progress in epidemiology and modeling
 BASIC RESEARCH TO IMPROVE UNDERSTANDING OF THE CORONAVIRUS
BASIC RESEARCH TO IMPROVE UNDERSTANDING OF THE CORONAVIRUS

Nearly a hundred research projects were launched over the year to shed light on the biology and structure of the SARS-CoV-2 virus, the organs it attacks, the inflammation and neurological signs it causes, the immune response triggered and any potential genetic susceptibility.
See Improving understanding of the biology of SARS-CoV-2 and COVID-19
 ANTIVIRAL STRATEGIES TO TREAT COVID-19
ANTIVIRAL STRATEGIES TO TREAT COVID-19

The Institut Pasteur launched several studies – searching for molecules involved in the key stages of the viral cycle, identifying those that target essential cell functions, conducting clinical trials and demonstrating the antiviral action of drugs already on the market – and set up a group to assess antiviral strategies.
See Avenues for therapeutic approaches
 VACCINE CANDIDATES TO PROTECT THE POPULATION AND CURB THE SPREAD OF THE EPIDEMIC
VACCINE CANDIDATES TO PROTECT THE POPULATION AND CURB THE SPREAD OF THE EPIDEMIC

Two research programs for vaccine candidates entered preclinical development in 2020 and are continuing in 2021: one vaccine using a lentiviral vector and one DNA vaccine.
The Institut Pasteur has decided not to pursue the clinical development of its vaccine candidate based on the measles platform, despite the Phase I clinical trials carried out on the vaccine last August, as the immune responses induced were not strong enough.
The scientists involved in the program will analyze the results obtained in more detail to try to understand why this was the case.
They will make further research proposals, some of which may be based on the same platform
See"Vaccine strategies" and Read the press release of january 25 2021
 SHARING INFORMATION WITH THE SCIENTIFIC COMMUNITY TO ADVANCE KNOWLEDGE MORE QUICKLY
SHARING INFORMATION WITH THE SCIENTIFIC COMMUNITY TO ADVANCE KNOWLEDGE MORE QUICKLY

On January 29, the Institut Pasteur sequenced the whole genome of SARS-CoV-2. The following day, it deposited the complete sequences of the virus samples taken from two of the first French cases on the GISAID (Global Initiative on Sharing All Influenza Data) platform. This transfer of information between scientists helps the international community to understand more about the evolution and spread of viruses.
Since April 1, every day from noon to midnight, the Institut Pasteur has contributed to the task of processing the many SARS-CoV-2 genomes submitted to GISAID (from dozens to hundreds every day worldwide) to validate the quality and reliability of the sequences and their metadata.
Again reflecting its ongoing efforts to advance knowledge, Institut Pasteur scientists deposited several preprints (preliminary versions of publications prior to acceptance by the peer review board of a scientific journal) throughout the year, both on the Institut Pasteur open archive and on international open science sites like medRxiv.org and bioRxiv.org.

__________________________________
All these major achievements are a testament to the unprecedented response from the Institut Pasteur's teams.
__________________________________
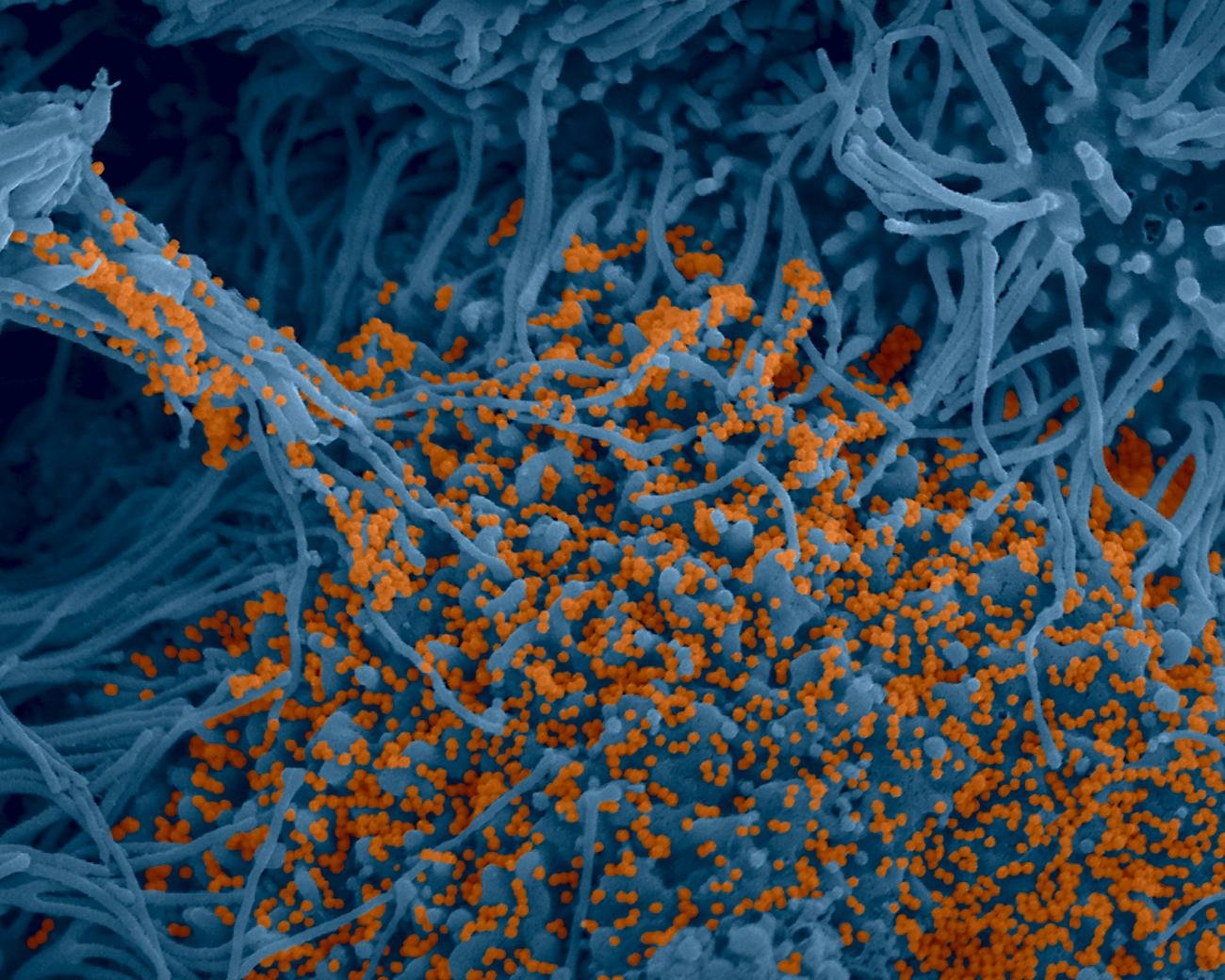
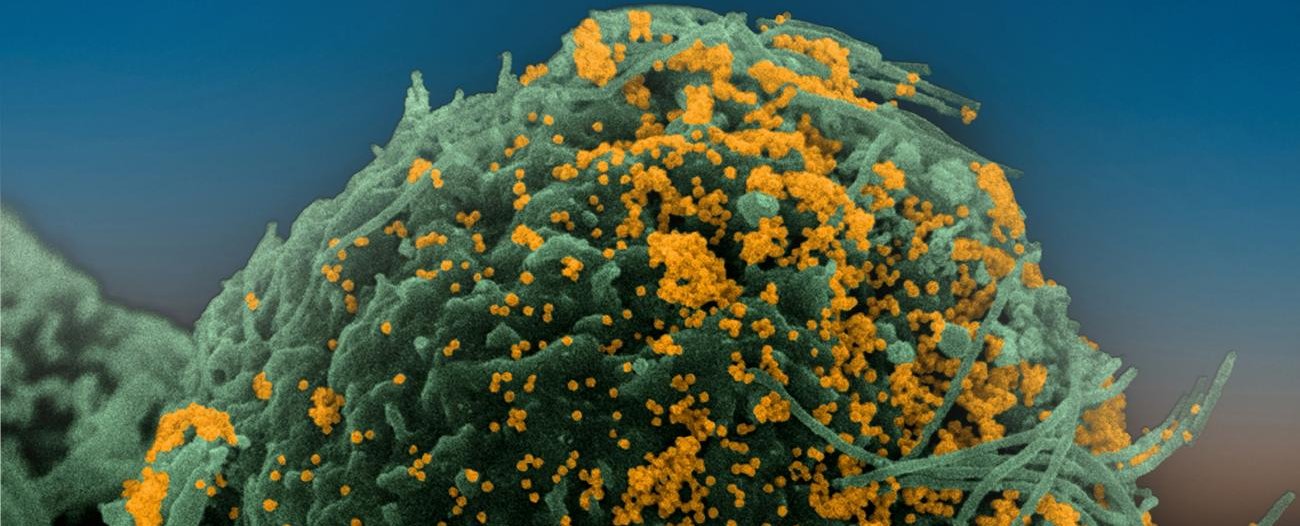
Institut Pasteur scientists use scanning electron microscopy to examine the virus’ strategy of attack. Shown here is a sample of bronchial cells grown in culture and colored in blue. In orange, the SARS-CoV-2 coronavirus.
Credit : Institut Pasteur. Image by Rémy Robinot, Mathieu Hubert, Vincent Michel, Olivier Schwartz and Lisa Chakrabarti, colorized by Jean Marc Panaud
- December 31, 2019 : the China Country Office of the World Health Organization (WHO) is informed that clustered cases of pneumonia of unknown etiology have been detected in the city of Wuhan, in the Chinese province of Hubei.
- From december 31, 2019 to January 3, 2020 : 44 patients with this pneumonia of unknown etiology are reported to WHO by the Chinese authorities. The pathogen causing this new disease has still not been identified (source WHO).
- January 7: informal discussions on the subject between various members of the Institut Pasteur Senior Management Board at the first meeting of the new year in January.
Le CNR des virus des infections respiratoires s’organise
- January 9 : chinese authorities and WHO raise the alarm
The CNR for Respiratory Viruses begins to monitor the novel coronavirus in France. This coronavirus is different from two other viruses known to have caused recent respiratory disease outbreaks: SARS-CoV, responsible for the SARS outbreak in 2003 (in February 2020 this virus was renamed "SARS-CoV-1"), and MERS-CoV, responsible for an outbreak that has been under way since 2012 in the Middle East. The Institut Pasteur was actively involved in tackling these previous outbreaks, which yielded valuable lessons for the current situation.

Sylvie van der Werf
Director of the National Reference Center (CNR) for Respiratory Infection Viruses at the Institut Pasteur.
For both SARS-CoV and MERS-CoV, cells known as Vero E6 cells were identified and can be used to culture the two coronaviruses. In January 2020, we brought them out of our collection, which is kept under strictly controlled conditions, so that we would be ready as soon as we detected a positive sample for the 2019-nCoV coronavirus.

Cytopathic effect of SARS-CoV-2 coronavirus on Vero E6 cells. Cell layer with a visible cytopathic effect (CPE); the cells attacked by the virus have been destroyed.
- January 12 :china shares the genetic sequence of the novel coronavirus (known for the time being as 2019-nCoV, for "novel coronavirus that emerged in 2019") so that countries can use it to develop specific diagnostic kits.
- January 13 : the Thai health authorities report the first laboratory-confirmed imported case of the novel coronavirus from China.
- Week of january 13 : the outbreak gains more media coverage and experts from the Institut Pasteur start replying to requests from the media.
- January 20 : health authorities in South Korea report the first case of novel coronavirus in the Republic of Korea. To date, 282 confirmed cases of 2019-nCoV have been reported to WHO by four countries: China (278 cases), Thailand (2 cases), Japan (1 case) and the Republic of Korea (1 case) (source WHO).
From January 20, WHO provides a daily report of all confirmed cases of infection with the novel coronavirus that are reported to it. - January 21 : a first Institut Pasteur COVID-19 fact sheet is made available online to inform the public. It is subsequently updated on a regular basis.
- January 22 : a first scientific consultation and leadership meeting is held in connection with the first priority research area of the Institut Pasteur's Strategic Plan, "emerging infectious diseases."

The Institut Pasteur has specialists in a wide range of fields and a depth of basic knowledge that paved the way for rapid progress in 2020.
Christophe d'Enfert leads the Coronavirus Task Force that was set up on January 23, 2020. The Task Force is composed of scientific experts from several disciplines and support staff from the Institut Pasteur's technical departments to ensure that scientific projects can be carried out as effectively as possible.
What have been the Institut Pasteur's strengths in dealing with the COVID-19 epidemic?
Above all, its ability to respond quickly, which is facilitated by its economic model – its status as a foundation greatly simplifies the launch of new projects. Many of the projects initiated this year were also made possible by the successful public fundraising appeal, which provided the funds needed to embark on major research in diagnostics, vaccine development, epidemiology and therapeutics back in mid-February, alongside the projects already started in January (see below Interview with Arnaud Fontanet). Without this outpouring of generous support, we would not have been able to launch as many projects. It was quite remarkable. The other strength is of course the expertise of our teams, which were immediately able to apply their knowledge to the novel coronavirus.
Can you give us some examples?
A thorough knowledge of viruses was crucial; we have carried out significant research in recent years on other coronaviruses, like SARS-CoV-1, which emerged in the early 2000s, then MERS-CoV. We also have technological expertise, such as vaccine strategies, which we were able to apply to the novel coronavirus. Another example is the expertise of one of our teams in identifying antibodies with therapeutic potential, which led to the rapid identification of drug candidates to treat COVID-19. We have specialists in a wide range of fields and a depth of basic knowledge that paved the way for relatively rapid progress. Scientists on campus with no links to virology also stepped up. For example, teams from the Department of Neuroscience started working on COVID-19 because of its association with neurological disorders such as loss of taste and smell.
How are things looking at the end of 2020?
Thanks partly to the generosity of the general public, we have funded dozens of projects and mobilized the efforts of nearly 400 people for multidisciplinary research on COVID-19 and SARS-CoV-2. Although we responded on a large scale in recent years to outbreaks of chikungunya, Ebola and Zika, our response to the COVID-19 pandemic has been quite unprecedented.
- January 24, late morning : the first samples (from the first imported French cases) arrive at the Institut Pasteur from Bichat Teaching Hospital. An RT-qPCR diagnostic test that had been under development by the CNR for Respiratory Infection Viruses, with information shared with the international community, is ready for use.
Detection of the virus is confirmed by the CNR the same evening. - January 25 : work to isolate the strains and sequence the viral genome begins at the Institut Pasteur.
- January 27, morning : the virus is isolated by the Institut Pasteur.
- January 28 : first meeting of the Task Force on the novel coronavirus involving leading scientists from the Institut Pasteur in each of the key disciplines and some support departments. From this point on, the Task Force meets every week and sets up thematic groups. The Task Force initiates the first scientific research programs launched by the Institut Pasteur from mid-February 2020.
Close-up of the whole SARS-CoV-2 coronavirus sequence in one of the first French cases, determined at the Institut Pasteur. The viral RNA bases can be seen. Credit : Institut Pasteur.
- January 30 :The Institut Pasteur is the first in Europe to sequence the whole genome of the novel coronavirus. The sequence is shared with the international scientific community via the GISAID website.
In parallel, an RT-qPCR diagnostic test is developed by the CNR for Respiratory Viruses. The RT-qPCR technique developed by the CNR for Respiratory Viruses, enabling highly sensitive, specific detection of the virus through nasopharyngeal swab specimens, was transferred to hospitals and made known to WHO so that it could be shared among the global network and with the scientific community as effectively as possible.
| « Whole-genome sequencing of the novel coronavirus is crucial to be able to develop specific diagnostic tests and identify potential treatment options. » Vincent Enouf, Deputy Director of the National Reference Center (CNR) for Respiratory Infection Viruses at the Institut Pasteur. |
- January 30 : In parallel, an RT-qPCR diagnostic test is developed by the CNR for Respiratory Viruses. The RT-qPCR technique developed by the CNR for Respiratory Viruses, enabling highly sensitive, specific detection of the virus through nasopharyngeal swab specimens, was transferred to hospitals and made known to WHO so that it could be shared among the global network and with the scientific community as effectively as possible.
The Institut Pasteur's historic mission is to fight emerging infectious diseases and make its innovations accessible to the entire world population.
This historic positioning and commitment was reaffirmed as early as 2014 at a workshop jointly organized by the Institut Pasteur (Paris) with the World Health Organization and the World Intellectual Property Organization. The principle is to encourage the transfer of technologies from academic research to industrial players through agreements aimed at guaranteeing accessibility of products and services to the greatest number, with free licenses for low-income countries.
This policy has been implemented particularly in response to the Covid-19 health crisis with an unprecedented mobilization of all the Institut Pasteur teams to provide diagnostic and vaccine solutions in record time; this in collaboration with national and international industrial partners to mass-produce these innovations and make them accessible as quickly as possible. Contracts for the exploitation of Pasteurian technologies include obligations for industrialists to :
- guarantee accessibility of the products to the greatest number of people;
- ensure distribution of the technologies in all countries;
- promote marketing at affordable prices depending on the situation in each country.
Some examples of the wide-ranging research at the Institut Pasteur :
• Development of an RT-qPCR diagnostic test by the CNR for Respiratory Infection Viruses
• Development of an RT-LAMP rapid diagnostic test
The Institut Pasteur's Laboratory for Urgent Response to Biological Threats (CIBU), which specializes in outbreak management, contributed its expertise together with key reagents to obtain a test that was as sensitive and specific as the RT-qPCR test but with a result in under 30 minutes. LAMP technology can be used outside medical test laboratories, for example in care homes, on ships or even in car parks. A consortium of industry partners was set up for the development of this test.
• Provision of serological tests to assess whether an individual has developed antibodies against SARS-CoV-2 proteins and therefore previously contracted the virus
These seroconversion tests can be used by epidemiologists to map the spread of the virus over a given area. They can also be used in conjunction with RT-qPCR tests in diagnosing patients. They are CE marked and approved by the US Food and Drug Administration.
• Phylogenomic studies showing the different introductions of the virus in France
• Rollout of assays using novel seroneutralization techniques to determine whether patients have developed neutralizing antibodies
These assays can also be used for diagnostic purposes, or to verify and confirm the performance of vaccines in development (via serum samples taken from vaccinated individuals). Another potential use is to ensure that specific populations, especially elderly people, have developed neutralizing antibodies following vaccination.
• Collaboration with diagnostic startups to develop innovative lateral flow antigen tests :
rapid results between 10 and 30 minutes, and higher sensitivity, reducing the need for RT-qPCR confirmation for those receiving a negative result (as is the case with current antigen tests).
As well as :
• Development of a diagnostic test by the Hong Kong University-Pasteur Research Pole and rollout in the International Network
• Bioinformatics Hub involved in curating genomes sequenced worldwide (GISAID) (Find out more)

Vincent ENOUF, deputy director of the Virus Influenzae national reference center at Institut Pasteur
Whole-genome sequencing of the novel coronavirus is crucial to be able to develop specific diagnostic tests and identify potential treatment options.
- January 31 :The Institut Pasteur holds a first press conference to announce the isolation and sequencing of coronavirus strains identified in France. During this press conference, a targeted fundraising campaign, "Coronavirus Emergency," is launched to support the Institut Pasteur's research.
- February 2 : The first death outside China is officially announced, in the Philippines (source WHO).
- February 11 :Forget the terms "Chinese flu" or "Wuhan coronavirus" – WHO announces that the disease responsible for the current epidemic is now called COVID-19 (for "coronavirus disease 2019"). The virus that causes the disease belongs to the family of SARS viruses and is now known as SARS-CoV-2 (an acronym derived from "severe acute respiratory syndrome coronavirus 2"). Why "2"? Because a first coronavirus (SARS-CoV-1) was already identified as the cause of a first epidemic that emerged in Hong Kong in 2002-2003.
- February 12 : A first SARS-CoV-2 call for scientific proposals is launched for Institut Pasteur staff (submissions are accepted on an ongoing basis until May 15, 2020).
- February 14 :First death caused by an indigenous case in France, related to the cluster in Crépy-en-Valois. Also on February 14, Stewart Cole, President of the Institut Pasteur, plays host to His Excellency Mr. Lu Shaye, Chinese Ambassador to France, on a visit to the Institut Pasteur to express his support both personally and on behalf of his country for the Institut Pasteur’s efforts in response to the novel coronavirus outbreak.
(See the article on the Research Journal). - Around February 20 : The epidemic spreads to Italy, where it progresses relatively quickly. On February 22, WHO reports 9 confirmed cases (source WHO). On February 23: 76 confirmed cases (source WHO). On February 28: 65 confirmed cases (source WHO). On March 1: 1,128 confirmed cases (source WHO).
- During the weekend February 22-23 : After the epidemic outbreak in China in January and February, the epidemic situation has developed at global level, with the escalation of outbreaks in South Korea, Japan and Singapore and the emergence of new outbreaks in Iran and Italy. These countries are witnessing community spread, with no identified link with cases imported from China.

- February 28 : WHO raises its assessment of the risk of spread and risk of impact of COVID-19 from "high" to "very high" at the global level (source WHO).
- March 4 :WHO notes that "Disruptions to the global supply of personal protective equipment (PPE) are leaving healthcare workers ill-equipped to care for patients" (source WHO).
- March 6 : One hundred thousand cases recorded worldwide.
In France, the Prime Minister prepares the public for the country's potential transition to phase 3 of the epidemic. If this happens, it will indicate that the coronavirus is circulating throughout the country and that the priority is to mitigate its impact, while treating the most severely affected patients. - March 7 : Launch of the first SARS-CoV-2 call for proposals for research projects to be implemented within the Institut Pasteur International Network (21 projects proposed by the International Network are selected by the Task Force out of a total of 36 proposals).
- From March 9 : A "COVID-19" internal scientific meeting is held at the Institut Pasteur every Monday. Twenty experts from the Institut Pasteur attend this first meeting; now the number attending the meetings is often more than a hundred.
- March 10 : All the countries in the European Union now have cases of COVID-19.
The Institut Pasteur senior management team informs its staff that it is preparing for the COVID-19 epidemic to potentially enter phase 3 (gradual transition to a reduced mode of operation, restriction of travel to at-risk countries, protection of sensitive populations, working from home, restriction of in-person meetings). A team responsible for the Business Continuity Plan (BCP) is set up.
- March 11 : WHO announces that the outbreak is now a pandemic.
- March 12 : In France, the President announces extraordinary measures for the entire country, to come into force on Monday March 16, including closing all schools and universities and asking companies to move to remote working.
- March 13: The statements by the French President and the progression of the outbreak lead the Institut Pasteur to take the decision to adopt a reduced mode of operation, to be implemented gradually from the beginning of the following week.
- March 14 : The French Prime Minister confirms that the epidemic has reached phase 3. The SARS-CoV-2 coronavirus is now circulating actively throughout France. The country is moving from a strategy of containment of the epidemic to a strategy of mitigation.
- March 16 : Start of the (first) lockdown in France.
- From March 17 : Nearly 400 scientists (out of a total of 2,780 staff members) are authorized to come onto the Institut Pasteur campus to continue research on SARS-CoV-2 and COVID-19. The Institut Pasteur is forced to impose remote working for most of its staff members who are able to work from home.
Combating Fake News
- March 17 : A misleading, defamatory video is posted online. Based on a misinterpretation of a patent filed in 2004, the video claims that the Institut Pasteur invented COVID-19 for commercial gain.
- March 17-20 : Given the viral nature of the initial erroneous information in social and mainstream media, a large-scale fact-checking campaign is launched and extensively shared via a wide variety of platforms (threads, videos, series, etc.) and by several institutions and official outlets. (Find out more)
- March 19 : The Coalition for Epidemic Preparedness Innovations (CEPI) funds the development of a SARS-CoV-2 vaccine based on a project led by a consortium composed of the Institut Pasteur, Themis and the University of Pittsburgh.
- March 20 : Launch of the website maladiecoronavirus.f (source, in french)
Maintaining “non-COVID” research activities

Anna-Bella Failloux
We did all we could not to lose the benefit of several years of work.
Anna-Bella Failloux leads the Arboviruses and Insect Vectors Unit at the Institut Pasteur in Paris, and with her team she manages an insectarium to breed mosquitoes that may be viral vectors (they may transmit viruses to humans). Large quantities of mosquitoes are bred in this vast facility in controlled conditions. The aim is to be in a position to respond to future challenges caused by emerging vector-borne diseases.
"On Monday March 16, 2020, we were told that we were going to have to close the laboratory. There are normally twelve of us in the team. On March 17, just four of us came in to look after the mosquitoes, to change them, feed them and carry out the necessary maintenance. For most of the species we house in the insectarium, daily maintenance is needed. If we hadn't been able to work as a smaller team, we would have lost all thirty mosquito populations, many of which come from our work in collaboration with the Institut Pasteur International Network.
The atmosphere was rather strange on campus as it was empty, but there was a real feeling of solidarity among Institut Pasteur staff. We were well looked after, we felt like we were valued. It was quite difficult, of course, but we managed to keep things going in that way. Because it is so important for the Institut Pasteur to maintain its "non-COVID" activities. It is one of the rare places in Europe with such a large number of research teams working in such varied fields, with international reach. There are so many pathogens that we need to continue investigating so that we can get to know them better and be in a better position to tackle them if and when they emerge."
Anna-Bella Failloux, Head of the Arboviruses and Insect Vectors Unit and the insectarium at the Institut Pasteur in Paris (Extract from the Pasteurdon conference on October 8, 2020)
- Late March : Scientists at the Institut Pasteur, with the support of the Hauts-de-France Regional Health Agency and the Amiens Education Authority and the backing of the French Blood Service (EFS), carry out an epidemiological survey on 661 people linked to a high school in Crépy-en-Valois, in the Oise department.
|
COMBATING FAKE NEWS
|
- April 10 : Launch of the second SARS-CoV-2 call for proposals within the International Network (for institutes in low- and middle-income countries).
- April 21 : Modeling carried out by the Institut Pasteur indicates that between 3% and 7% of French people have been infected (source)

- April 23 :Following the epidemiological survey carried out in Crépy-en-Valois and the use of virus detection tests together with three serological tests developed by the Institut Pasteur, a study reveals that 26% of the surveyed population have been infected by SARS-CoV-2 and developed antibodies against the virus. The population continues to be monitored and other studies are successively published.
- April 30 : The Institut Pasteur informs its staff about preparations for the implementation of the Progressive Business Resumption Plan (PBRP) on campus from May 11, after the French government announces the start date for the gradual easing of lockdown measures.
- April 30 : An international initiative comprising scientists from the University of California, San Francisco (UCSF), the Gladstone Institutes, the Icahn School of Medicine at Mount Sinai and the Institut Pasteur reveals the identification of promising compounds for clinical trials with the aim of tackling COVID-19. (source)
Wide-ranging research at the Institut Pasteur. Some examples :
- Creation of a group to evaluate antiviral strategies proposed by teams from the Institut Pasteur and also from academia and industry
The idea was to respond to the many requests from private and academic groups in France and abroad wanting to find out whether certain small molecules or biological molecules (e.g. proteins or antibodies) were capable of blocking SARS-CoV-2 in a variety of cellular models. A working group evaluated the relevance of the 56 requests received and accepted 32 of them (15 from industry and 17 from academia).
The aim of these analyses was to draw on the Institut Pasteur's expertise for the identification of novel therapies by the rapid development of models suited to specific viruses during pandemics, in this case the COVID-19 pandemic.
The work enabled several private and public laboratories to confirm or refute scientific theories as part of their efforts to find therapeutic solutions to treat the virus.
At the end of the process, a small number of molecules were found to have an effect, sometimes a powerful one, on this type of cellular model. The findings will have to be confirmed with subsequent analyses on more advanced models, some of which will involve research partnerships with the Institut Pasteur.
- Identifying molecules and antibodies that target key stages in the viral cycle: fusion, replication and maturation
(see the press release: "Innate immunity and fusion of cells infected with SARS-CoV-2" )
- International cooperation to identify molecules targeting cellular functions with an essential role in the viral cycle (UCSF and Mount Sinai)
And also :
- Clinical studies: evaluation of chemoprophylactic approaches for healthcare workers
- Demonstration of the antiviral action of drugs that already have market approval (niclosamide) and launch of clinical trials by the Institut Pasteur Korea
- Early May : More than 120 scientists are registered for the internal COVID-19 scientific meetings at the Institut Pasteur. Meetings are held via video conference. Scientific work on COVID-19 at the Institut Pasteur is now divided among twelve working groups.
Specific shared tools are developed to optimize discussions and boost cooperation. - May 7 : Following its first internal call for scientific proposals, 38 research projects led by the Institut Pasteur in Paris are selected by the Task Force out of a total of 55 proposals submitted.
- May 11 : Gradual easing of lockdown measures begins in France.
Wide-ranging research at the Institut Pasteur. Some examples :
- Modeling the epidemic and the consequences of lockdown on its progression (see "COVID-19: mathematical model indicates that between 3% and 7% of French people have been infected").
- Development of serological assays and tests to detect neutralizing antibodies (see "Development and evaluation of four serological assays to detect SARS-CoV-2 antibodies and two assays to detect neutralizing antibodies").
- Epidemiological studies (see the interview with Arnaud Fontanet).
- Characteristics of community transmission (Crépy-en-Valois).
- Establishment and persistence of the immune response in symptomatic cases (Curie-O-SA, Crépy).
- Nosocomial transmission (see Clinical Infectious Diseases "A Conceptual Discussion About the Basic Reproduction Number of Severe Acute Respiratory Syndrome Coronavirus 2 in Healthcare Settings").
- Telemedicine and new technologies (see Use of the maladiecoronavirus.fr web application).
- May 14 : A serological assay using bioluminescence (known as LuLISA), developed by the Institut Pasteur, increases the sensitivity of detection of specific immunoglobulins and is shown to be effective in allergy assessment. The LuLISA assay is adapted to detect antibodies (immunoglobulins IgG, IgM, IgA and IgE) directed against proteins of the SARS-CoV-2 coronavirus, which causes COVID-19. The Institut Pasteur files a patent application for LuLISA (source)
- Mid-May : A seroprevalence study in the French population, led by Santé publique France, reveals that the frequency of seropositives is 5% and the frequency of neutralizing antibodies is 3.5%.
The serological assays developed by the Institut Pasteur teams (LuLISA N, LuLISA S and pseudoneutralization) are used in this study - June :The Curie-O-SA study, led by the Institut Curie in collaboration with the Institut Pasteur, will examine the long-term dynamics of humoral, cellular and mucosal immune responses in staff from the Institut Curie and the Institut Pasteur who have been infected by SARS-CoV-2.
- June 29 : An epidemiological study led by several Institut Pasteur teams in primary schools in Crépy-en-Valois since April, involving 1,340 people, does not reveal significant transmission between children or from children to teachers, and confirms that children are more often infected by adults at home (source)
"The Institut Pasteur was actively involved in Crépy-en-Valois, with Inserm, in two epidemiological studies carried out by Santé publique France"

Arnaud Fontanet
The Institut Pasteur was actively involved in Crépy-en-Valois, with Inserm, in two epidemiological studies carried out by Santé publique France
Two questions to Arnaud Fontanet, Director of the Department of Global Health and Head of the Epidemiology of Emerging Diseases Unit at the Institut Pasteur.
The Institut Pasteur carried out large-scale studies on the COVID-19 cluster that emerged in Crépy-en-Valois in February 2020. Tell us more.
"Yes, we got involved when the cluster emerged in Crépy-en-Valois. We first became aware of the cluster when a middle school teacher died on February 25 in Paris. Working with Santé publique France, we carried out a study on March 5-6 which revealed that the virus was circulating actively in Oise and which also allowed us to take samples that we could use to develop serological assays. These samples were complemented by the samples from the national cohort led by Inserm, meaning that several teams on campus were able to develop serological assays based on various technologies, which we reported on in a scientific publication. As well as developing different types of tests, my team carried out two epidemiological studies in Crépy that enabled us to document an outbreak that had occurred in a high school in the town in the first fortnight of February and to confirm the fact that the virus was circulating in primary schools but without any major outbreaks."
What were the results of these studies in the high school and primary schools?
"For the high school, we demonstrated that the virus was circulating actively: 41% of people who had been in contact with the high school, whether students, teachers or other adults, had been infected. We then tried to find out whether the outbreak had also occurred in primary schools in Crépy-en-Valois. The first finding of our study was that children aged between 6 and 11 had had mild forms of the disease, and that 44% of them had been asymptomatic. The virus had spread more in families, from parents and siblings to children rather than the other way round. In the period that we studied, there were three introductions of the virus in primary schools without any cases of secondary transmission. We got the impression, but it remains to be proven, that children under the age of 11 are slightly less susceptible to infection, unlike high school students, who behave like adults and contract more visible, transmissible forms. We also saw that children who had been infected by other seasonal coronaviruses that had caused colds were not protected against the novel coronavirus."
- Before summer 2020 (northern hemisphere) : Circulation of the virus has fallen considerably in countries that have brought their first wave under control, but this is mainly because of the stringent measures taken to control the virus.
- July : The Institut Pasteur Task Force launches a further call for proposals, aimed at all the teams in the Institut Pasteur International Network, for ambitious, multidisciplinary research projects carried out over several years that will involve recruiting contract researchers. The proposals will be assessed in autumn and will begin in early 2021.
- July 15 : Thanks to the discovery of an association between a type 1 interferon deficiency in the blood and severe COVID-19 forms, early detection and a potential therapeutic approach can be offered to at-risk patients (source)
- July 24 : An intranasal lentiviral vaccine in development provides significant protection in animals (source)
- August :The Phase I clinical trial to assess the safety and immunogenicity of vaccine candidate TMV-083 (also known as V591, previously MV-SARS-CoV-2) begins in France. This is the first time the vaccine candidate, developed by Institut Pasteur scientists in partnership with the companies Themis and MSD with the support of CEPI, has been administered to humans.
- Summer 2020 : A slowdown in the circulation of the virus is observed in the northern hemisphere, probably because the virus is being transmitted less between people who are spending time outside in summer. The virus continues circulating at a low level.
- September 17 : Thirty million cases and 930,000 deaths have been recorded worldwide.
Since the beginning of 2020, the Institut Pasteur has been working on several vaccines for the SARS-CoV-2 virus, which causes COVID-19. Some examples :
- Seven vaccine strategies were under assessment in autumn 2020 at the Institut Pasteur, five for the induction of humoral responses (antibodies, spike protein) and two for the induction of cytotoxic responses.
- Collaboration with industry stakeholders at the national and international level has been vital to speed up the development of six of these vaccine candidates.
- Significant progress was made on three research programs in particular in 2020; one of these unfortunately had to be discontinued in January 2021 but work on the other two is continuing (see below).
- Development of animal models.
Two vaccine candidates at the end of preclinical stage
Significant progress was made on three research programs in particular in 2020; one of these unfortunately had to be discontinued in January 2021 but work on the other two is continuing.
A vaccine using a lentiviral vector
This vaccine, designed for nasal administration, demonstrated good efficacy and extremely high production of antibodies in preclinical trials. The vaccine, developed with the biotech company Theravectys, is composed of a lentiviral vector. Lentiviruses are "slow" viruses (from the Latin lentus, meaning slow), characterized by a long incubation period before they become pathogenic. The virus is genetically modified to make it harmless for humans and to enable it to produce the spike protein.
- With Theravectys.
A DNA vaccine
Of the various SARS-CoV-2 vaccines developed at the Institut Pasteur, the DNA vaccine is undoubtedly the one that uses the most cutting-edge technology. The principle is to inject a DNA molecule into human cells. These cells recognize the DNA molecule and transcribe it into an RNA molecule capable of producing the SARS-CoV-2 spike protein. This protein, which forms spikes all around the virus, is the entry key allowing the virus into the cell.
- With In-Cell-Art.
A vaccine using the measles vector
Following the intermediate results of the Phase I clinical trial, the Institut Pasteur stopped development of this vaccine candidate on January 25, 2021. The vaccine candidate was an attenuated live virus vaccine using the measles vaccine as a vector (or vehicle) and expressing a spike protein antigen from the SARS-CoV-2 virus (the protein that serves as the entry key allowing the virus into cells).
- Phase I in August 2020 in France and Belgium
- Trial stopped in January 2021 due to unsatisfactory intermediate results
- CEPI funding: fair access
- Themis – MSD/Merck partnership
The decision to stop the clinical trial has no adverse impact on the continuation of research to tackle the SARS CoV-2 virus, including that based on this platform using the measles vaccine virus as a vaccine vector, or the continuation of other vaccine research projects being conducted in partnership with Themis/Merck-MSD, with this same platform, and aimed at other infectious diseases (Lassa fever and chikungunya). A vaccine candidate for chikungunya is currently in Phase III clinical trials (the results of the Phase I and II trials were published in The Lancet).

Photo en microscopie à balayage électronique. En jaune, le coronavirus SARS-CoV-2
- September : An electron microscope photo of the coronavirus, taken by the Institut Pasteur, is published (see above).
- September 29 :A study based on the Institut Pasteur’s modeling work shows how the lockdown has affected the COVID-19 epidemic in regions of mainland France. The lockdown appears to have succeeded in reducing the burden on intensive care units in the most severely affected regions, and in preventing epidemic outbreaks in other regions (source)
- October 6 : Lung-on-a-chip research shows the effect of SARS-CoV-2 on ciliated cells (on biorxiv).
- October 7 : The 14th edition of Pasteurdon is held against the backdrop of the COVID-19 epidemic (source, in french)
|
COMBATING FAKE NEWS
|
Wide-ranging research at the Institut Pasteur. Some examples :
- Biology of SARS-CoV-2 (see the effect of SARS-CoV-2 on cells), see the video below.
Video microscopy below showing syncytium formation following SARS-CoV-2 infection.
- Structural biology : Study of the viral cycle in cellula using cryo-electron microscopy, interactions between the S protein, receptors and antibodies
- Tropism : lungs, digestive tract, neurons (organs-on-chips)
- Cell biology : intercellular traffic, interference with host functions (miRNA)
- COVID-19 and host responses
- Humoral and cellular responses
- Transition between viral and inflammatory disease (see "In severe and critical patients, a severely impaired response of interferon (IFN) type I is associated with a persistent blood viral load and an excessive inflammatory response" at www.pasteur.fr)
- COVID-19 and neurological signs (in partnership with the Brain and Spine Institute)
- COVID-19 and genetic susceptibility
- Autumn 2020 : A new wave of the epidemic, feared by experts, is observed in the northern hemisphere, especially in Europe and the United States.
- October 13 : Scientists from the Institut Pasteur and the CNRS set out to investigate the consequences of SARS-CoV-2 infection for cell function and the antiviral role of innate immunity. Using real-time video microscopy, they show that infected cells in culture can fuse with neighboring cells and then die. But interferon counters this phenomenon by inducing cellular proteins that prevent the fusion of infected cells (See the press releas « Innate immunity and fusion of cells infected with SARS-CoV-2 »).
- October 15 : An international team of scientists (including members of the Institut Pasteur) identifies common vulnerabilities in the SARS-CoV-2, SARS-CoV-1 and MERS-CoV coronaviruses. (source)
- October 21 : Based on its analysis of specimens from more than 11,000 individuals, the CNR for Respiratory Infection Viruses provides an estimate of the seroprevalence of SARS-CoV-2 antibodies in France (national serological surveillance conducted with Santé publique France): approximately 5% of French people had antibodies in mid-May, but only 70% of individuals testing positive for SARS-CoV-2 had detectable neutralizing antibodies (source)
- October 28 : The French President announces a second lockdown in France.
- October 29 : The Institut Pasteur introduces new measures to reduce the presence of staff on campus, giving priority to scientists whose wet-lab research activities require laboratory access and extending remote working to all staff able to do so.
|
COMBATING FAKE NEWS
|
- November 3 : The number of deaths from COVID-19 in under-65s is a more reliable indicator for evaluating infection rates in populations, according to the findings of an Institut Pasteur study demonstrating that a simple comparison of the total number of deaths between countries could offer a misleading picture of the actual level of SARS-CoV-2 transmission.

- November 15 : A study conducted on hospital staff who contracted a mild form of the disease caused by SARS-CoV-2 seem to suggest that immunity lasts longer in women than in men (source ; on MedRxiv).
- November 18 : Initial results are published concerning the link between SARS-CoV-2 and the nervous system; COVID-19-associated olfactory dysfunction reveals SARS-CoV-2 neuroinvasion and persistence in the olfactory system (on biorxiv.org).
- November 22 : The efficacy of ivermectin (a drug used to treat parasitic conditions such as scabies) on certain COVID-19 symptoms is proven in golden hamsters (on biorxiv.org).
- November 23 : An analysis of data obtained from the website maladiecoronavirus.fr and the application COVIDOM shows that these self-assessment tools can reduce the load for call centers and predict peaks in hospital admissions. A study demonstrates that use of the web application maladiecoronavirus.fr, a tool developed in partnership with the Institut Pasteur, led to an eightfold reduction in the number of unnecessary calls to the French medical emergency number (15) (source).
- December 1st : First results of a serological survey (Curie-O-SA) conducted in May on 1,850 volunteers from the staff of the Institut Curie, in collaboration with the Institut Pasteur (use of the LuLISA assay, see "May 14"). The results from this representative sample of the working population in the Greater Paris region reveal a high prevalence of immunization and a relatively short-lived immune response. (Read the press release « First results of a large scale study conducted among Institut Curie staff on the immune response to SARS-CoV-2 », in french).
- December 8 : The results of a study reveal the importance of tailoring surveillance strategies to facilities’ testing capacities. (Read the press release « Optimizing surveillance in long-term care facilitiese »).
- December 17 : The Institut Pasteur, in partnership with the French National Health Insurance Fund (CNAM), Santé publique France and the Ipsos Social Research Institute, present the results of the ComCor epidemiological study on circumstances and places of infection with the SARS-CoV-2 virus (read the press release «ComCor: where are French people catching the virus »).
Sources of the numbers : Department of scientific affairs (SGS), Scientific Information Resources Center (CeRIS), Research Applications and Industrial Relations Department (DARRI), Institut Pasteur.